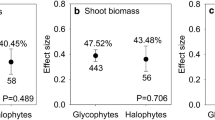
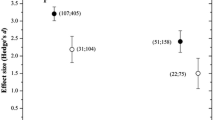
Abstract
Salt stress limits crop yield and sustainable agriculture in most arid and semiarid regions of the world. Arbuscular mycorrhizal fungi (AMF) are considered bio-ameliorators of soil salinity tolerance in plants. In evaluating AMF as significant predictors of mycorrhizal ecology, precise quantifiable changes in plant biomass and nutrient uptake under salt stress are crucial factors. Therefore, the objective of the present study was to analyze the magnitude of the effects of AMF inoculation on growth and nutrient uptake of plants under salt stress through meta-analyses. For this, data were compared in the context of mycorrhizal host plant species, plant family and functional group, herbaceous vs. woody plants, annual vs. perennial plants, and the level of salinity across 43 studies. Results indicate that, under saline conditions, AMF inoculation significantly increased total, shoot, and root biomass as well as phosphorous (P), nitrogen (N), and potassium (K) uptake. Activities of the antioxidant enzymes superoxide dismutase, catalase, peroxidase, and ascorbate peroxidase also increased significantly in mycorrhizal compared to nonmycorrhizal plants growing under salt stress. In addition, sodium (Na) uptake decreased significantly in mycorrhizal plants, while changes in proline accumulation were not significant. Across most subsets of the data analysis, identities of AMF (Glomus fasciculatum) and host plants (Acacia nilotica, herbs, woody and perennial) were found to be essential in understanding plant responses to salinity stress. For the analyzed dataset, it is concluded that under salt stress, mycorrhizal plants have extensive root traits and mycorrhizal morphological traits which help the uptake of more P and K, together with the enhanced production of antioxidant enzymes resulting in salt stress alleviation and increased plant biomass.







Similar content being viewed by others
References
Abogadallah GM (2010) Antioxidative defense under salt stress. Plant Signal Behav 5:369–374
Alberton O, Kuyper TW, Gorissen A (2005) Taking mycocentrism seriously: mycorrhizal fungal and plant responses to elevated CO2. New Phytol 167:859–868
Alguacil MM, Hernandez JA, Caravaca F, Portillo B, Roldan A (2003) Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiol Plant 118:562–570
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173:808–816
Bonfante P, Genre A (2010) Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat Commun 1:1–11
Borde M, Dudhane M, Jite PK (2010) AM fungi influences the photosynthetic activity, growth and antioxidant enzymes in Allium sativum L. under salinity condition. Not Sci Biol 2(4):64–71
Borde M, Dudhane M, Jite PK (2011) Growth photosynthetic activity and antioxidant responses of mycorrhizal and non-mycorrhizal bajra (Pennisetum glaucum) crop under salinity stress condition. Crop Prot 30:265–271
Borowicz VA (2001) Do arbuscular mycorrhizal fungi alter plant–pathogen relations? Ecology 82:3057–3068
Bothe H (2012) Arbuscular mycorrhiza and salt tolerance of plants. Symbiosis 58:7–16
Chatzistathis T, Orfanoudakis M, Alifragis D, Therios I (2013) Colonization of Greek olive cultivars’ root system by arbuscular mycorrhiza fungus: root morphology, growth, and mineral nutrition of olive plants. Sci Agric 70(3):185–194
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681
Colmer TD, Epstein E, Dvorak J (1995) Differential solute regulation in leaf blades of various ages in salt-sensitive wheat and a salt-tolerant wheat x Lophopyrum elongatum (host) A. Löve amphiploid. Plant Physiol 108:1715–1724
Copas J, Shi JQ (2000) Meta-analysis, funnel plots and sensitivity analysis. Biostatistics (Oxf Engl 1:247–262
Daei G, Ardekani MR, Rejali F, Teimuri S, Miransari M (2009) Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J Plant Physiol 166:617–625
Dodd IC, Pérez-Alfocea F (2012) Microbial amelioration of crop salinity stress. J Exp Bot 63:3415–3428
Dudhane M, Borde M, Jite PK (2011) Effect of Arbuscular Mycorrhizal fungi on growth and antioxidant activity in Gmelina arborea Roxb. under salt stress condition. Not Sci Biol 3(4):71–78
Estrada B, Aroca R, Maathuis JM, Barea JM, Ruiz-lozano JM (2013) Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ 36:1771–1782
Evelin H, Kapoor R, Giri B (2009) Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot 104:1263–1280
Evelin H, Giri B, Kapoor R (2012) Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22:203–217
Fitter AH, Helgason T, Hodge A (2011) Nutritional exchanges in the arbuscular mycorrhizal symbiosis: implications for sustainable agriculture. Fungal Biol Rev 25:68–72
Frechill S, Lasa B, Ibarretxe L, Lamsfus C, Aparicio Trejo P (2001) Pea response to saline stress is affected by the source of nitrogen nutrition (ammonium or nitrate). Plant Growth Regul 35:171–179
Fusconi A (2013) Regulation of root morphogenesis in arbuscular mycorrhizae: what role do fungal exudates, phosphate, sugars and hormones play in lateral root formation? Ann Bot. doi:10.1093/aob/mct258
Garg N, Chandel S (2011) The effects of salinity on nitrogen fixation and trehalose metabolism in mycorrhizal Cajanus cajan (L.) Mill sp. plants. J Plant Growth Regul 30:490–503
Garg N, Chandel S (2012) Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Mill sp. under NaCl and Cd stresses. J Plant Growth Regul 31:292–308
Garg N, Manchanda G (2008) Effect of arbuscular mycorrhizal inoculation of salt-induced nodule senescence in Cajanus cajan (pigeonpea). J Plant Growth Regul 27:115–124
Garg N, Manchanda G (2009) Role of arbuscular mycorrhizae in the alleviation of ionic, osmotic and oxidative stresses induced by salinity in Cajanus cajan (L.) Mill sp. (pigeonpea). J Agron Crop Sci 195:110–123
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Giri B, Mukerji KG (2004) Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 14:307–312
Giri B, Kapoor R, Mukerji KG (2007) Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb Ecol 54:753–760
Gosset DR, Millhollon EP, Lucas MC (1994) Antioxidant response to NaCl stress in salt tolerant and salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Gurevitch J, Hedges LV (1999) Statistical issues in ecological meta-analysis. Ecology 80:1142–1149
Gurevitch J, Curtis PS, Jones MH (2001) Meta-analysis in ecology. Adv Ecol Res 32:199–247
Hajiboland R, Aliasgharzadeh N, Laiegh SF, Poschenrieder C (2010) Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 331:313–327
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hoeksema JD, Bala Chaudhary V, Gehring CA, Collins Johnson N, Karst J, Koide RT, Pringle A, Zabinski C, Bever JD, Moore JC, Wilson GWT, Klironomos JN, Umbanhowar J (2010) A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol Lett 13:394–407
Jahromi F, Aroca R, Porcel R, Ruiz-Lozano JM (2008) Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microb Ecol 55:45–53
Jansa J, Mozafar A, Frossard E (2005) Phosphorus acquisition strategies within arbuscular mycorrhizal fungal community of a single field site. Plant Soil 276:163–176
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Jayne B, Quigley M (2014) Influence of arbuscular mycorrhiza on growth and reproductive response of plants under water deficit: a meta-analysis. Mycorrhiza 24:109–119
Juniper S, Abbott LK (2006) Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16:371–379
Kaya C, Ashraf M, Sonmez O, Aydemir S, Tuna AL, Cullu MA (2009) The influence of arbuscular mycorrhizal colonization on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci Hortic 121:1–6
Ketchum REB, Scott Warren R, Klima LJ, Francisco Lopez-Gutierrez F, Nabors MW (1991) The mechanism and regulation of proline accumulation in suspension cultures of the halophytic grass Distichlis spicata L. J Plant Physiol 137:368–374
Kohler J, Caravaca F, Roldán A (2010) An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol Biochem 42:429–434
Koricheva J, Gange AC, Jones T (2009) Effects of mycorrhizal fungi on insect herbivores: a meta-analysis. Ecology 90:2088–2097
Lekberg Y, Koide RT (2005) Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol 168:189–204
Maiale S, Sanchez DH, Guirado A, Vidal A, Ruiz OA (2004) Spermine accumulation under salt stress. J Plant Physiol 161:35–42
Mayerhofer MS, Kernaghan G, Harper KA (2013) The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23:119–128
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, Marion JL, Mitchell CE, Parker IM, Power AG, Torchin ME, Vazguez DP (2007) Direct and interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology 88:1021–1029
Munkvold L, Kjøller R, Vestberg M, Rosendahl S, Jakobsen I (2004) High functional diversity within species of arbuscular mycorrhizal fungi. New Phytol 164:357–364
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Patel D, Saraf M (2013) Influence of soil ameliorants and microflora on induction of antioxidant enzymes and growth promotion of Jatropha curcas L. under saline condition. Eur J Soil Biol 55:47–54
Porcel R, Aroca R, Ruiz-Lozano JM (2012) Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron Sustain Dev 32:181–200
Rengasamy P (2006) World salinization with emphasis on Australia. J Exp Bot 57:1017–1023
Rinaldelli E, Mancuso S (1996) Response of young mycorrhizal and non-mycorrhizal plants of olive tree (Olea europaea L.) to saline conditions. I. Short-term electrophysiological and long-term vegetative salt effects. Adv Hortic Sci 10:126–134
Rosenberg NJ, Adams DC, Gurevitch J (2000) Metawin: statistical software for meta-analysis version 2.0. Sinauer, Sunderland
Sannazzaro AI, Echeverria M, Albertó EO, Ruiz OA, Menéndez AB (2007) Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol Biochem 45:39–46
Schellenbaum L, Berta G, Ravolanirina F, Tisserant B, Gianinazzi S, Fitter AH (1991) Influence of endomycorrhizal infection on root morphology in a micropropagated woody plant species (Vitis vinifera L.). Ann Bot 68:135–141
Serrano R, Rodriguez-Navarro A (2001) Ion homeostasis during salt stress in plants. Curr Op Cell Biol 13:399–404
Sharifi M, Ghorbanli M, Ebrahimzadeh H (2007) Improved growth of salinity-stressed soybean after inoculation with pre-treated mycorrhizal fungi. J Plant Physiol 164:1144–1151
Sharma MP, Gaur A, Bhatia NP, Adholeya A (1996) Growth responses and dependence of Acacia nilotica var. cupriciformis on the indigenous arbuscular mycorrhizal consortium of a marginal wasteland soil. Mycorrhiza 6:441–446
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012: 10.1155/2012/217037
Sheng M, Tang M, Chen H, Yang B, Zhang F, Huang Y (2008) Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18:287–296
Smith FA, Jakobsen I, Smith SE (2000) Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytol 147:357–366
Smith SE, Smith FA, Jakobsen I (2004) Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol 162:511–524
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Talaat NB, Shawky BT (2013) Modulation of the ROS-scavenging system in salt-stressed wheat plants inoculated with arbuscular mycorrhizal fungi. J Plant Nutr Soil Sci. doi:10.1002/jpln.201200618
Talaat NB, Shawky BT (2014) Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot 98:20–31
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Tian CY, Feng G, Li XL, Zhang FS (2004) Different effects of arbuscular mycorrhizal fungal isolates from saline or non-saline soil on salinity tolerance of plants. App Soil Ecol 26:143–148
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK (2013) The extent of mycorrhizal colonization of roots and its influence on plant growth and phosphorus content. Plant Soil 371:1–13
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.)-differential response in salt-tolerant and sensitive varieties. Plant Sci 165:1411–1418
van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecol 79:2082–2091
Veresoglou SD, Menexes G, Rillig MC (2012) Do arbuscular mycorrhizal fungi affect the allometric partition of host plant biomass to shoots and roots? A meta-analysis of studies from 1990 to 2010. Mycorrhiza 22:227–235
Worchel ER, Hannah E, Giauque, Kivlin SN (2013) Fungal symbionts alter plant drought response. Microb Ecol 65:671–678
Wu QS, Zou YN, He XH (2010) Contributions of arbuscular mycorrhizal fungi to growth, photosynthesis, root morphology and ionic balance of citrus seedlings under salt stress. Acta Physiol Plant 32:297–304
Wu QS, Zou YN, He XH (2013) Mycorrhizal symbiosis enhances tolerance to NaCl stress through selective absorption but not selective transport of K+ over Na+ in trifoliate orange. Sci Hortic 160:366–374
Zhang JL, Flowers TJ, Wang SM (2010) Mechanisms of sodium uptake by roots of higher plants. Plant Soil 326:45–60
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MEST) (No. 2012R1A2A1A01005294).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chandrasekaran, M., Boughattas, S., Hu, S. et al. A meta-analysis of arbuscular mycorrhizal effects on plants grown under salt stress. Mycorrhiza 24, 611–625 (2014). https://doi.org/10.1007/s00572-014-0582-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-014-0582-7