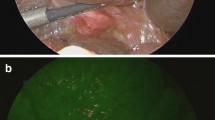
Abstract
Thoracic surgeons perform a wide variety of cancer operations, which are often associated with high morbidity and mortality. Thus, thoracic surgery involves many special challenges that require innovative solutions. The increased utilization of minimally invasive practices, poor overall cancer survival, and significant morbidity of critical operations remain key obstacles to overcome. Fluorescence imaging technology (FIT), involving the implementation of fluorescent dyes and imaging systems, is currently used as an adjunct for general thoracic surgery in many situations and includes sentinel lymph node mapping, pulmonary intersegmental plane identification, pulmonary nodule identification, pulmonary bullous lesion detection, evaluation of the anastomotic perfusion after tracheal surgery, and thoracic duct imaging for postoperative chylothorax. This technology enhances the surgeon’s ability to perform operations, and has specific advantages. We review some of the key studies that demonstrate the applications of FIT in the field of general thoracic surgery, focusing on the use of indocyanine green.

Similar content being viewed by others
References
Okusanya OT, Hess NR, Luketich JD, Sarkaria IS. Infrared intraoperative fluorescence imaging using indocyanine green in thoracic surgery. Eur J Cardiothorac Surg. 2018;53:512–8.
Moody ED, Viskari PJ, Colyer CL. Non-covalent labeling of human serum albumin with indocyanine green: a study by capillary electrophoresis with diode laser-induced fluorescence detection. J Chromatogr B Biomed Sci Appl. 1999;729:55–64.
De Grand AM, Lomnes SJ, Lee DS, Pietrzykowski M, Ohnishi S, Morgan TG, et al. Tissue-like phantoms for near-infrared fluorescence imaging system assessment and the training of surgeons. J Biomed Opt. 2006;11:014007.
De Jesus E, Keating JJ, Kularatne SA, Jiang J, Judy R, Predina J, et al. Comparison of folate receptor targeted optical contrast agents for intraoperative molecular imaging. Int J Mol Imaging. 2015;2015:469047.
O’Shannessy DJ, Yu G, Smale R, Fu YS, Singhal S, Thiel RP, et al. Folate receptor alpha expression in lung cancer: Diagnostic and prognostic significance. Oncotarget. 2012;3:414–25.
Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–84.
Dosio F, Milla P, Cattel L. EC-145, a folate-targeted Vinca alkaloid conjugate for the potential treatment of folate receptor-expressing cancers. Curr Opin Investig Drugs. 2010;11:1423–33.
Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–62.
Ebel W, Routhier EL, Foley B, Jacob S, McDonough JM, Patel RK, Turchin HA, et al. Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immun. 2007;7:6.
Spannuth WA, Sood AK, Coleman RL. Farletuzumab in epithelial ovarian carcinoma. Expert Opin Biol Ther. 2010;10:431–7.
Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–95.
Chilvers AS, Thomas MH. Methods for the localization of incompetent ankle perforating veins. Br Med J. 1970;2:577–9.
Feindel W, Yamamoto YL, Hodge CP. Red cerebral veins and the cerebral steal syndrome. Evidence from fluorescein angiography and microregional blood flow by radioisotopes during excision of an angioma. J Neurosurg. 1971;35:167–79.
Ross AJ 3rd, O'Neill JA Jr, Silverman DG, Brousseau DA, Gatti JE, Templeton JM Jr. A new technique for evaluating cutaneous vascularity in complicated conjoined twins. J Pediatr Surg. 1985;20:743–6.
Keating JJ, Okusanya OT, De Jesus E, Judy R, Jiang J, Deshpande C, et al. Intraoperative molecular imaging of lung adenocarcinoma can identify residual tumor cells at the surgical margins. Mol Imaging Biol. 2016;18:209–18.
Hoogstins CE, Tummers QR, Gaarenstroom KN, de Kroon CD, Trimbos JB, Bosse T, et al. A novel tumor-specific agent for intraoperative near-infrared fluorescence imaging: a translational study in healthy volunteers and patients with ovarian cancer. Clin Cancer Res. 2016;22:2929–38.
Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58:R37–61.
Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol. Imaging. 2010;9:237–55.
Pikin O, Filonenko E, Mironenko D, Vursol D, Amiraliev A. Fluorescence thoracoscopy in the detection of pleural malignancy. Eur J Cardiothorac Surg. 2012;41:649–52.
Veronesi U, Paganelli G, Galimberti V, Viale G, Zurrida S, Bedoni M, et al. Sentinel-node biopsy to avoid axillary dissection in breast cancer with clinically negative lymph-nodes. Lancet. 1997;349:1864–7.
Albertini JJ, Cruse CW, Rapaport D, Wells K, Ross M, DeConti R, et al. Intraoperative radio-lympho-scintigraphy improves sentinel lymph node identification for patients with melanoma. Ann Surg. 1996;223:217–24.
Thompson JF, McCarthy WH, Bosch CM, O'Brien CJ, Quinn MJ, Paramaesvaran S, et al. Sentinel lymph node status as an indicator of the presence of metastatic melanoma in regional lymph nodes. Melanoma Res. 1995;5:255–60.
Tiffet O, Nicholson AG, Khaddage A, Prévot N, Ladas G, Dubois F, et al. Feasibility of the detection of the sentinel lymph node in peripheral non-small cell lung cancer with radio isotopic and blue dye techniques. Chest. 2005;127:443–8.
Schmidt FE, Woltering EA, Webb WR, Garcia OM, Cohen JE, Rozans MH. Sentinel nodal assessment in patients with carcinoma of the lung. Ann Thorac Surg. 2002;74:870–5.
Ito N, Fukuta M, Tokushima T, Nakai K, Ohgi S. Sentinel node navigation surgery using indocyanine green in patients with lung cancer. Surg Today. 2004;34:581–5.
Yamashita S-I, Tokuishi K, et al. Sentinel node navigation surgery by thoracoscopic fluorescence imaging system and molecular examination in non-small cell lung cancer. Ann Surg Oncol. 2012;19:728–33.
Yamashita S, Tokuishi K, Anami K, Miyawaki M, Moroga T, Kamei M, et al. Video-assisted thoracoscopic indocyanine green fluorescence imaging system shows sentinel lymph nodes in non-small-cell lung cancer. J Thorac Cardiovasc Surg. 2011;141:141–4.
Gilmore DM, Khullar OV, Jaklitsch MT, Chirieac LR, Frangioni JV, Colson YL. Identification of metastatic nodal disease in a phase 1 dose escalation trial of intraoperative sentinel lymph node mapping in nonsmall cell lung cancer using near-infrared imaging. J Thorac Cardiovasc Surg. 2013;146:562–70.
Nomori H, Cong Y, Sugimura H. Utility and pitfalls of sentinel node identification using indocyanine green during segmentectomy for cT1N0M0 non-small cell lung cancer. Surg Today. 2016;46:908–13.
Yuasa Y, Seike J, Yoshida T, Takechi H, Yamai H, Yamamoto Y, et al. Sentinel lymph node biopsy using intraoperative indocyanine green fluorescence imaging navigated with preoperative CT lymphography for superficial esophageal cancer. Ann Surg Oncol. 2012;19:486–93.
Hachey KJ, Gilmore DM, Armstrong KW, Harris SE, Hornick JL, Colson YL, et al. Safety and feasibility of near-infrared image-guided lymphatic mapping of regional lymph nodes in esophageal cancer. J Thorac Cardiovasc Surg. 2016;152:546–54.
Schlottmann F, Barbetta A, Mungo B, Lidor AO, Molena D. Identification of the lymphatic drainage pattern of esophageal cancer with nearinfrared fluorescent imaging. J Laparoendosc Adv Surg Tech A. 2017;27:268–71.
Helminen O, Mrena J, Sihvo E. Near-infrared image-guided lymphatic mapping in minimally invasive oesophagectomy of distal oesophageal cancer. Eur J Cardiothorac Surg. 2017;52:952–7.
Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg. 1960;39:555–72.
Bedetti B, Bertolaccini L, Rocco R, Schmidt J, Solli P, Scarci M. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2017;9:1615–23.
Nomori H, Shiraishi A, Cong Y, Sugimura H, Mishima S. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg. 2018;53:640–7.
Okada M, Mimura T, Ikegaki J, Katoh H, Itoh H, Tsubota N. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg. 2007;133:753–8.
Oizumi H, Kato H, Endoh M, Inoue T, Watarai H, Sadahiro M. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg. 2014;97:1456–8.
Sekine Y, Ko E, Oishi H, Miwa M. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg. 2012;143:1330–5.
Oh S, Suzuki K, Miyasaka Y, Matsunaga T, Tsushima Y, Takamochi K. New technique for lung segmentectomy using indocyanine green injection. Ann Thorac Surg. 2013;95:2188–90.
Sekine Y, Itoh T, Toyoda T, et al. Precise anatomical sublobar resection using a 3d medical image analyzer and fluorescence-guided surgery with transbronchial instillation of indocyanine green. Semin Thorac Cardiovasc Surg. 2019;S1043–0679:30413–21.
Misaki N, Chang SS, Gotoh M, Yamamoto Y, Satoh K, Yokomise H. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg. 2009;138:613–8.
Misaki N, Chang SS, Igai H, Tarumi S, Gotoh M, Yokomise H. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg. 2010;140:752–6.
Tarumi S, Misaki N, Kasai Y, Chang SS, Go T, Yokomise H. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg. 2014;46:112–5.
Chen R, Ma Y, Li C, et al. A pilot study of pulmonary segmentectomy with indocyanine green near-infrared angiography. Surg Innov. 2019;26:337–43.
Mehta M, Patel YS, Yasufuku K, et al. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg. 2019;157:2029–35.
Mun M, Okumura S, Nakao M, Matsuura Y, Nakagawa K. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg. 2017;3:80.
Matsuura Y, Mun M, Ichinose J, Nakao M, Nakagawa K, Okumura S. Recent fluorescence-based optical imaging for video-assisted thoracoscopic surgery segmentectomy. Ann Transl Med. 2019;7:32.
Blackmon SH, Feinglass SR. The United States preventive services task force recommendations for lung cancer screening. Thorac Surg Clin. 2015;25:199–203.
Cerfolio RJ, Bryant AS, McCarty TP, Minnich DJ. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg. 2011;91:1696–701.
Ujiie H, Kato T, Hu HP, Patel P, Wada H, Fujino K, et al. A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: A phase I feasibility trial. J Thorac Cardiovasc Surg. 2017;154:702–11.
Anayama T, Qiu J, Chan H, Nakajima T, Weersink R, Daly M, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg. 2015;99:224–30.
Abbas A, Kadakia S, Ambur V, Muro K, Kaiser L. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg. 2017;153:1581–90.
Hachey KJ, Digesu CS, Armstrong KW, Gilmore DM, Khullar OV, Whang B, et al. A novel technique for tumor localization and targeted lymphatic mapping in early-stage lung cancer. J Thorac Cardiovasc Surg. 2017;154:1110–8.
Okusanya OT, Holt D, Heitjan D, Deshpande C, Venegas O, Jiang J, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg. 2014;98:1223–300.
Kim HK, Quan YH, Choi BH, Park JH, Han KN, Choi Y, et al. Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur J Cardiothorac Surg. 2016;49:1497–502.
Kawakita N, Takizawa H, Sawada T, Matsumoto M, Tsuboi M, Toba H, et al. Indocyanine green fluorescence imaging for resection of pulmonary metastasis of hepatocellular carcinoma. J Thorac Dis. 2019;11:944–9.
Kitagawa N, Shinkai M, Mochizuki K, Usui H, Miyagi H, Nakamura K, et al. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr Surg Int. 2015;31:407–11.
Keating J, Newton A, Venegas O, Nims S, Zeh R, Predina J, et al. Near-infrared intraoperative molecular imaging can locate metastases to the lung. Ann. Thorac. Surg. 2016;5:391–403.
Predina JD, Newton AD, Corbett C, Shin M, Sulfyok LF, Okusanya OT, et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J Thorac Cardiovasc Surg. 2019;157:2061–9.
Hamaji M, Chen-Yoshikawa TF, Minami M, Date H. Near-infrared imaging using intravenous indocyanine green at a conventional dose to locate pulmonary metastases: a pilot study. Thorac Cardiovasc Surg. 2018. https://doi.org/10.1055/s-0038-1675346.
Horio H, Nomori H, Fuyuno G, Kobayashi R, Suemasu K. Limited axillary thoracotomy vs video-assisted thoracoscopic surgery for spontaneous pneumothorax. Surg Endosc. 1998;12:1155–8.
Gotoh M, Okamoto T, Yamamoto Y, Liu D, Kameyama K, Hayashi E, et al. Development of a canine model of pulmonary emphysema and imaging of the emphysematous lung with infrared thoracoscopy. J Thorac Cardiovasc Surg. 2003;126:1916–21.
Gotoh M, Yamamoto Y, Igai H, Chang S, Huang C, Yokomise H. Clinical application of infrared thoracoscopy to detect bullous or emphysematous lesions of the lung. J Thorac Cardiovasc Surg. 2007;134:1498–501.
Li H, Zhou J, Chi C, Mao Y, Yang F, Tian J, et al. Clinical application of near-infrared thoracoscope with indocyanine green in video-assisted thoracoscopic bullectomy. J Thorac Dis. 2016;8:1841–5.
Matsumoto K, Sano I, Taniguchi H, Yamasaki N, Tsuchiya T, Miyazaki T, et al. Thoracoscopic surgery for lung emphysema using an infrared camera. J Cardiothorac Surg. 2013;8:134.
Schweiger T, Schwarz S, Traxler D, Dodier P, Aigner C, Lang G, et al. Bronchoscopic indocyanine green fluorescence imaging of the anastomotic perfusion after tracheal surgery. Ann Thorac Surg. 2016;101:1943–9.
Akin H, Olcmen A, Isgorucu O, Denizkiran I, Dincer I. Approach to patients with chylothorax complicating pulmonary resection. Thorac Cardiovasc Surg. 2012;60:135–9.
Ashitate Y, Tanaka E, Stockdale A, Choi HS, Frangioni JV. Near-infrared fluorescence imaging of thoracic duct anatomy and function in open surgery and video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 2011;142:31–38.e1–2
Kamiya K, Unno N, Konno H. Intraoperative indocyanine green fluorescence lymphography, a novel imaging technique to detect a chyle fistula after an esophagectomy: report of a case. Surg Today. 2009;39:421–4.
Kaburagi T, Takeuchi H, Oyama T, Nakamura R, Takahashi T, Wada N, et al. Intraoperative fluorescence lymphography using indocyanine green in a patient with chylothorax after esophagectomy: report of a case. Surg Today. 2013;43:206–10.
Matsutani T, Hirakata A, Nomura T, Hagiwara N, Matsuda A, Yoshida H, et al. Transabdominal approach for chylorrhea after esophagectomy by using fluorescence navigation with indocyanine green. Case Rep Surg. 2014;2014:1–4.
Shirotsuki R, Uchida H, Tanaka Y, Shirota C, Yokota K, Murase N, et al. Novel thoracoscopic navigation surgery for neonatal chylothorax using indocyanine-green fluorescent lymphography. J Pediatr Surg. 2018;53:1246–9.
Yang F, Zhou J, Li H, Yang F, Xiao R, Chi C, et al. Near-infrared fluorescence-guided thoracoscopic surgical intervention for postoperative chylothorax. Interact Cardiovasc Thorac Surg. 2018;26:171–5.
Bethea BT, Okamura AM, Kitagawa M, Fitton TP, Cattaneo SM, Gott VL, et al. Application of haptic feedback to robotic surgery. J Laparoendosc Adv Surg Tech A. 2004;14:191–5.
Tholey G, Desai JP, Castellanos AE. Force feedback plays a significant role in minimally invasive surgery: Results and analysis. Ann Surg. 2005;241:102–9.
Hagen ME, Meehan JJ, Inan I, Morel P. Visual clues act as a substitute for haptic feedback in robotic surgery. Surg Endosc. 2008;22:1505–8.
Kitagawa M, Dokko D, Okamura AM, Yuh DD. Effect of sensory substitution on suture-manipulation forces for robotic surgical systems. J Thorac Cardiovasc Surg. 2005;29:151–8.
Okusanya OT, DeJesus EM, Jiang JX, Judy RP, Venegas OG, Deshpande CG, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg. 2015;150:28–35 e1.
Funding
This research was funded by the Cancer Institute Hospital of Japanese Foundation for Cancer Research (No. 2010-1055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We have no conflicts of interest to declare.
Ethical approval
This research was approved by the Institutional Review Board of Clinical Research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Matsuura, Y., Ichinose, J., Nakao, M. et al. Recent fluorescence imaging technology applications of indocyanine green in general thoracic surgery. Surg Today 50, 1332–1342 (2020). https://doi.org/10.1007/s00595-019-01906-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-019-01906-6