Abstract
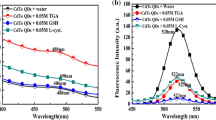
CdS quantum dots (QDs) have been prepared and modified with chitosan. Based on the quenching of fluorescence signals of the functionalized CdS QDs at 531 nm wavelength and enhancement of signals the 400–700 nm wavelength range by Cu2+ at pH 4.2, a simple, rapid and specific method for Cu2+ determination is presented. Under optimum conditions, the relative fluorescence intensity of CdS QDs is linearly proportional to copper concentration from 8.0 nmol L−1 to 3.0 μmol L−1 with a detection limit of 1.2 nmol L−1. The mechanism can be explained in terms of strong binding of Cu2+ onto the surface of CdS, resulting in a chemical displacement of Cd2+ ions and the formation of CuS on the surface of the QDs.







Similar content being viewed by others
References
Murray CB, Norris DJ, Bawendi MG (1993) Synthesis and characterization of nearly monodisperse CdE (E = S, Se, Te) semiconductor nanocrystallites. J Am Chem Soc 115:8706
Peng ZA, Peng XG (2001) Formation of high-quality CdTe, CdSe, and CdS nanocrystals using CdO as precursor. J Am Chem Soc 123:183
Stinaff EA, Scheibner M, Bracker AS et al (2006) Optical signatures of coupled dots. Science 311:636
Clapp AR, Medintz IL, Uyeda HT (2005) Quantum dot-based multiplexed fluorescence resonance energy transfer. J Am Chem Soc 127:18212
Goldman ER, Balighian ED, Mattoussi H et al (2002) Avidin: a natural bridge for quantum dot-antibody conjugates. J Am Chem Soc 124:6378
Larson DR, Zipfel WR, Williams RM et al (2003) Water-soluble quantum dots for multiphoton fluorescence imaging in Vivo. Science 300:1434
Giemans BNG, Adams SR, Ellisman MH et al (2006) The fluorescent toolbox for assessing protein location and function. Science 312:217
Chen YF, Rosenzweig Z (2002) Luminescent CdS quantum dots as selective ion probes. Anal Chem 74:5132
Gatas-Asfura KM, Leblanc RM (2003) Peptide-coated CdS quantum dots for the optical detection of copper(II) and silver. Chem Commun (21):2684
Chen JL, Zhu CQ (2005) Functionalized cadmium sulfide quantum dots as fluorescence probe for silver ion determination. Anal Chim Acta 546:147
Li HB, Zhang Y, Wang XQ, Gao ZN (2008) A luminescent nanosensor for Hg(II) based on functionalized CdSe/ZnS quantum dots. Microchim Acta 160:119
Wu HM, Liang JG, Han HY (2008) A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim Acta 161:81
Lai SJ, Chang XJ, Mao J et al (2007) Determination of silver ion with cadmium sulfide quantum dots modified by bismuthiol II as fluorescence probe. Ann Chim- Rome 97:109
Yi H, Wu LQ, Bentley WE et al (2005) Biofabrication with chitosan, biomacromolecules. Biomacromolecules 6:2881
Krajewska B (2001) Diffusion of metal ions through gel chitosan membranes. React Funct Polym 47:37
Li Z, Du YM, Zhang ZL et al (2003) Preparation and characterization of CdS quantum dots chitosan biocomposite. React Funct Polym 55:35
Demas JN, Crosby GA (1971) The measurement of photoluminescence quantum yields. J Phys Chem A 75:991
Yu WW, Qu LH, Guo WZ et al (2003) Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 15:2854
Kolhe P, Kannan RM (2003) Improvement inductility of chitosan through blending and copolymerization with PEG: FTIR investigation of molecular interaction. Biomacromolecules 4:173
Cao YC, Wang JH (2004) One-pot synthesis of high-quality zinc-blend CdS nanocrystals. J Am Chem Soc 126:14336
Sarov AV, Chrysochoos J (1997) Optical and photochemical properties of nonstoichiometric cadmium sulfide nanoparticles: surface modification with copper(II) ions. Langmuir 13:3142
Gao M, Kirstein S, Mhwald H et al (1998) Strongly photoluminescent CdTe nanocrystals by proper surface modification. J Phys Chem 102:8360
Florence TM (1982) The speciation of trace elements in waters. Talanta 29:345
Myung N, Bae Y, Bard AJ (2003) Enhancement of the photoluminescence of CdSe nanocrystals dispersed in CHCl3 by oxygen passivation of surface states. Nano Lett 3:747
Liang JG, Ai XP, He ZK et al (2004) Functionalized CdSe quantum dots as selective silver ion chemodosimeter. Analyst 129:619
Costa-Fernández JM, Pereiro R, Sanz-Medel A (2006) The use of luminescent quantum dots for optical sensing. Trac- Trend Anal Chem 25:207
Klostranec JM, Chan WCW (2006) Quantum dots in biological and biomedical research: recent progress and present challenges. Adv Mater 18:1953
Xie HY, Liang JG, Zhang ZL, Liu Y (2004) Luminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochimica Acta A 60:2527
Cao QE, Wang KT, Hu ZD et al (1998) Syntheses of three new derivatives of 8-aminoquinoline and its applications for fluorimetric determination of copper(II). Talanta 47:921
Kawakubo S, Kato H, Watsuki MI (1994) Catalytic spectrofluorimetric determination of copper using aerial oxidation of ascorbic acid in the presence of o-phenylenediamine. Analyst 119:2119
Torsten M, Dorota W, Tobias W (2001) Fluorimetric determination of copper(II) in aqueous solution using lucifer yellow CH as selective metal reagent. Fresenius J Anal Chem 371:44
Chen B, Zhong P (2005) A new determining method of copper(II) ions at ng ml−1 levels based on quenching of the water-soluble nanocrystals fluorescence. Anal Bioanal Chem 381:986
Acknowledgements
This work was financially supported by the personal introducement program of the Chongqing Three Gorges University (2007-SXX-YRC-006), Chongqing, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, S., Chang, X. & Fu, C. Cadmium sulfide quantum dots modified by chitosan as fluorescence probe for copper (II) ion determination. Microchim Acta 165, 39–44 (2009). https://doi.org/10.1007/s00604-008-0094-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-008-0094-2