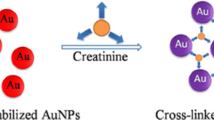
Abstract
We describe a highly sensitive method for the determination of traces of xanthine based on the aggregation of citrate-stabilized gold nanoparticles (AuNPs). It is found that, under optimal conditions of pH, the imide group of xanthine is adsorbed on the surface of the AuNPs, thereby displacing citrate ions. This leads to an aggregation of the AuNPs via hydrogen-bond interactions. As a result, the color of the solution changes from red to blue which can be seen with bare eyes and also can be measured by spectrophotometry. The ratio of the absorbances at 630 nm and 520 nm is linearly related to the concentration of xanthine in the 125 nM to 6.0 μM range (r = 0.9988), and the detection limit (3σ/slope) is 23 nM. The method is simple, feasible and fast.

We describe a highly sensitive colorimetric method for the determination of traces of xanthine based on the aggregation of citrate-stabilized gold nanoparticles (AuNPs). The ratio of the absorbances at 630 nm and 520 nm is linearly related to the concentration of xanthine in the 125 nM to 6.0 μM range, and the detection limit is 23 nM. The method is simple, feasible and fast.






Similar content being viewed by others
References
Luong JHT, Male KB, Nguyen AL (1989) Application of polarography for monitoring the fish postmortem metabolite transformation. Enzyme Microb Technol 11:277–282
Campion EW, Glynn RJ, Delabry LO (1987) Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med 82:421–426
Kim KY, Ralph Schumacher H, Hunsche E, Wertheimer AI, Kong SX (2003) A literature review of the epidemiology and treatment of acute gout. Clin Ther 25:1593–1617
Gok F, Ichida K, Topaloglu R (2003) Mutational analysis of the xanthine dehydrogenase gene in a Turkish family with autosomal recessive. Nephrol Dial Transplant 18:2278–2283
Banupriya C, Ratnakar DP, Mondal N, Vishnu B, Koner BC (2008) Can urinary excretion rate of malon-dealdehyde, uric acid and protein predict the severity and impending death in perinatal asphyxia. Clin Biochem 41:968–973
McMaster-Fay RA (2008) Pre-eclampsia-a disease of oxidative stress resulting from the catabolism of DNA (primarily fetal). Biosci Hypotheses 1:35–43
Carsol MA, Volpe G, Mascini M (1997) Amperometric detection of uric acid and hypoxanthine with Xanthine oxidase immobilized and carbon based screen-printed electrode. Application for fish freshness determination. Talanta 44:2151–2159
Mu S, Shi Q (2013) Xanthine biosensor based on the direct oxidation of xanthine at an electrogenerated oligomer film. Biosens Bioelectron 47:429–435
Zhang L, Lei J, Zhang J, Ding L, Ju H (2012) Amperometric detection of hypoxanthine and xanthine by enzymatic amplification using a gold nanoparticles- carbon nanohorn hybrid as the carrier. Analyst 137:3126–3131
Devi R, Yadav S, Pundir CS (2012) Amperometric determination of xanthine in fish meat by zinc oxide nanoparticle/chitosan/multiwalled carbon nanotube/ polyaniline composite film bound xanthine oxidase. Analyst 137:754–759
Pei J, Li XY (2000) Xanthine and hypoxanthine sensors based on xanthine oxidaseimmobilized on a CuPtCl6 chemically modified electrode and liquid chromatog-raphy electrochemical detection. Anal Chim Acta 414:205–213
Schultz DA (2003) Plasmon resonant particles for biological detection. Curr Opin Biotechnol 14:13–22
Li H, Rothberg LJ (2004) Label-Free colorimetric detection of specific sequences in genomic DNA amplified by the polymerase chain reaction. J Am Chem Soc 126:10958–10961
Spain E, Kojima R, Kaner RB, Wallace GG, O’Grady J, Lacey K, Barry T, Keyes TE, Forster RJ (2011) High sensitivity DNA detection using gold nanoparticle functionalised polyaniline nanofibres. Biosens Bioelectron 26:2613–2618
Woravith C, Apichat I (2012) Visual and colorimetric detection of mercury (II) ion using gold nanoparticles stabilized with a dithia-diaza ligand. Microchim Acta 176:57–64
Pu WD, Zhao HW, Huang CZ, Wu LP, Xu D (2013) Visual detection of arginine based on the unique guanidino group-induced aggregation of gold nanoparticles. Anal Chim Acta 18:78–83
Li W, Nie Z, He K, Xu X, Li Y, Huang Y, Yao S (2011) Simple, rapid and label-free colorimetric assay for Zn2+ based on unmodified gold nanoparticles and specific Zn2+ binding peptide. Chem Commun 47:4412–4414
Ghosh SK, Pal T (2007) Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem Rev 107:4797–4862
Wang XX, Wu Q, Shan Z, Huang QM (2011) BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens Bioelectron 15:3614–3619
Xu D, Zhao HW, Huang CZ, Wu LP, Pu WD, Zheng JJ, Zuo Y (2011) Sensitive and selective detection of mercury (II) based on the aggregation of gold nanoparticles stabilized by riboflavin. J Nanosic Nanotechnol 12:3006–3010
Jin R, Wu G, Li Z, Mirkin CA, Schatz GC (2003) What controls the melting properties of DNA-linked gold nanoparticle assemblies. J Am Chem Soc 125:1643–1654
Roy B, Saha A, Nandi AK (2011) Melamine sensing through riboflavin stabilized gold nanoparticles. Analyst 136:67–70
Ling J, Sang Y, Huang CZ (2008) Visual colorimetric detection of berberine hydrochloride with silver nanoparticles. J Pharmaceut Biomed 47:860–864
Kulikowska E, Kierdaszuk B, Shugar D (2004) Xanthine, xanthosine and its nucleotides: solution structures of neutral and ionic forms, and relevance to substrate properties in various enzyme systems and metabolic pathways. Acta Biochim Pol 51:493–531
Bera RK, Anoop A, Raj CR (2011) Enzyme-free colorimetric assay of serum uric acid. Chem Commun 47:11498–11500
Kthiwala M, Affum AO, Perry J, Brajter-Toth A (2008) Direct measurements of xanthine in 2000-fold diluted xanthinuric urine with a nanoporous carbon fiber sensor. Analyst 33:810–816
Acknowledgments
This work was supported by the Chongqing Natural Science Foundation (CSTC, 2007BB0049) and the National Natural Science Foundation Committee of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pu, W., Zhao, H., Wu, L. et al. A colorimetric method for the determination of xanthine based on the aggregation of gold nanoparticles. Microchim Acta 182, 395–400 (2015). https://doi.org/10.1007/s00604-014-1342-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1342-2