Abstract
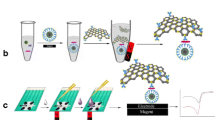
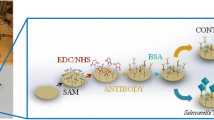
This article describes an electrochemical immunosensor for rapid determination of Salmonella pullorum and Salmonella gallinarum. The first step in the preparation of the immunosensor involves the electrodeposition of gold nanoparticles used for capturing antibody and enhancing signals. In order to generate a benign microenvironment for the antibody, the ionic liquid (IL) 1-butyl-3-methylimidazolium hexafluorophosphate was used to modify the surface of a screen-printed carbon electrode (SPCE). The single steps of modification were monitored via cyclic voltammetry and electrochemical impedance spectroscopy. Based on these findings, a sandwich immunoassay was worked out for the two Salmonella species by immobilizing the respective unlabeled antibodies on the SPCE. Following exposure to the analytes, secondary antibody (labeled with HRP) is added to form the sandwich. After adding hydrogen peroxide and thionine, the latter is oxidized and its signal measured via CV. A linear response to the Salmonella species is obtained in the 104 to 109 cfu · mL−1 concentration range, and the detection limits are 3.0 × 103 cfu · mL−1 for both species (at an SNR of 3). This assay is sensitive, highly specific, acceptably accurate and reproducible. Given its low detection limit, it represents a promising tool for the detection of S. pullorum, S. gallinarum, and - conceivably - of other food-borne pathogens by exchanging the antibody.

We describe an electrochemical sandwich assay based on a screen-printed carbon electrode, gold nanoparticles and ILs and capable of detecting Salmonella pullorum and Salmonella gallinarum. The preparation is outlined in the Schematic.









Similar content being viewed by others
References
Hong SS (2013) Therapeutic effects of bacteriophages against salmonella gallinarum infection in chickens. J Microbiol Biotechnol 23:1478–1483
Barrow PA, Neto OCF (2011) Pullorum disease and fowl typhoid—new thoughts on old diseases: a review. Avian Pathol 40:1–13
Batista DFA, De Freitas Neto OC, Lopes PD et al (2013) Polymerase chain reaction assay based on ratA gene allows differentiation between salmonella enterica subsp. Enterica serovar gallinarum biovars gallinarum and pullorum. J Vet Diagn Investig 25:259–262
Van Immerseel F, Studholme DJ, Eeckhaut V et al (2013) Salmonella Gallinarum field isolates from laying hens are related to the vaccine strain SG9R. Vaccine 31:4940–4945
Soria MC, Soria MA, Bueno DJ et al (2013) Comparison of 3 culture methods and PCR assays for salmonella gallinarum and salmonella pullorum detection in poultry feed. Poult Sci 92:1505–1515
Roda A, Mirasoli M, Roda B et al (2012) Recent developments in rapid multiplexed bioanalytical methods for foodborne pathogenic bacteria detection. Microchim Acta 178:7–28
Baumler AJ, Tsolis RM, Ficht TA et al. (1998) Evolution of host adaptation in Salmonella enterica. Infect Immun 66
Hu CM, Dou WC, Zhao GY (2014) Enzyme immunosensor based on gold nanoparticles electroposition and streptavidin-biotin system for detection of S. Pullorum & S. Gallinarum. Electrochim Acta 117:239–245
Bäumler AJ, Hargis BM, Tsolis RM (2000) Tracing the origins of Salmonella outbreaks. Science 287:50–52
Wang D, Dou WC, Zhao GY et al (2014) Immunosensor based on electrodeposition of gold-nanoparticles and ionic liquid composite for detection of Salmonella pullorum. J Microbiol Methods 106:110–118
Hu X, Dou WC, Fu LL et al (2013) A disposable immunosensor for Enterobacter sakazakii based on an electrochemically reduced graphene oxide-modified electrode. Anal Biochem 434:218–220
Zhao GY, Zhan XJ, Dou WC (2011) A disposable immunosensor for Shigella flexneri based on multiwalled carbon nanotube/sodium alginate composite electrode. Anal Biochem 408:53–58
Zhan XJ, Tang WL, Dou WC et al (2013) Dispoable immunosensor for Escherichia Coli O157:H7 based on a multi-walled carbon nanobon nanotube sodium alginate nanocomposite film modified screen-printed carbon electrode. Anal Lett 46:2690–2704
Toh SY, Citartan M, Gopinath SC et al (2015) Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens Bioelectron 64:392–403
Centi S, Messina G, Tombelli S et al (2008) Different approaches for the detection of thrombin by an electrochemical aptamer-based assay coupled to magnetic beads. Biosens Bioelectron 23:1602–1609
Omidfar K, Zarei H, Gholizadeh F et al (2012) A high-sensitivity electrochemical immunosensor based on mobile crystalline material-41-polyvinyl alcohol nanocomposite and colloidal gold nanoparticles. Anal Biochem 421:649–656
Regiart M, Pereira SV, Spotorno VG et al (2013) Nanostructured voltammetric sensor for ultra-trace anabolic drug determination in food safety field. Sensors Actuators B Chem 188:1241–1249
Wang XJ, Li XJ, Luo CN et al (2014) Ultrasensitive molecularly imprinted electrochemical sensor based on magnetism graphene oxide/beta-cyclodextrin/Au nanoparticles composites for chrysoidine analysis. Electrochim Acta 130:519–525
Derkus B, Emregul E, Emregul KC et al (2014) Alginate and alginate-titanium dioxide nanocomposite as electrode materials for anti-myelin basic protein immunosensing. Sensors Actuators B Chem 192:294–302
Chen X, Qin P, Li J et al (2014) Impedance immunosensor for bovine interleukin-4 using an electrode modified with reduced graphene oxide and chitosan. Microchim Acta 182:369–376
Li Y, Liu X, Zeng X et al (2009) Simultaneous determination of ultra-trace lead and cadmium at a hydroxyapatite-modified carbon ionic liquid electrode by square-wave stripping voltammetry. Sensors Actuators B Chem 139:604–610
Attri P, Jha I, Choi EH et al (2014) Variation in the structural changes of myoglobin in the presence of several protic ionic liquid. Int J Biol Macromol 69:114–123
Satoshi Shimano HZ, Itaru H (2007) Preparation of nanohybrid solid-state electrolytes with liquidlike mobilities by solidifying ionic liquids with silica particles. Am Chem Soc 19:6
Li R, Xia Q, Li Z et al (2013) Electrochemical immunosensor for ultrasensitive detection of microcystin-LR based on graphene-gold nanocomposite/functional conducting polymer/gold nanoparticle/ionic liquid composite film with electrodeposition. Biosens Bioelectron 44:235–240
Kumar A, Rani A, Venkatesu P et al (2014) Quantitative evaluation of the ability of ionic liquids to offset the cold-induced unfolding of proteins. Phys Chem Chem Phys: PCCP 16:15806–15810
Du P, Liu S, Wu P et al (2007) Preparation and characterization of room temperature ionic liquid/single-walled carbon nanotube nanocomposites and their application to the direct electrochemistry of heme-containing proteins/enzymes. Electrochim Acta 52:6534–6547
Feng R, Zhang Y, Yu HQ et al (2013) Nanoporous PtCo-based ultrasensitive enzyme-free immunosensor for zeranol detection. Biosens Bioelectron 42:367–372
Fang Y-S, Chen S-Y, Huang X-J et al (2014) Simple approach for ultrasensitive electrochemical immunoassay of clostridium difficile toxin B detection. Biosens Bioelectron 53:238–244
Guo AP, Li YY, Cao et al (2015) An electrochemical immunosensor for ultrasensitive detection of carbohydrate antigen 199 based on Au@CuxOS yolk-shell nanostructures with porous shells as labels. Biosens Bioelectron 63
Fan HX, Zhang Y, Wu D et al (2013) Construction of label-free electrochemical immunosensor on mesoporous carbon nanospheres for breast cancer susceptibility gene. Anal Chim Acta 770:62–67
Dong J, Zhao H, Xu M et al (2013) A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem 141:1980–1986
Olsen EV, Pathirana ST, Samoylov AM et al (2003) Specific and selective biosensor for Salmonella and its detection in the environment. J Microbiol Meth 53:273–285
Nguyen P-D, Tran TB, Nguyen DTX et al (2014) Magnetic silica nanotube-assisted impedimetric immunosensor for the separation and label-free detection of Salmonella typhimurium. Sensors Actuators B Chem 197:314–320
Si SH, Lia X, Fung YS, Zhu DR (2001) Rapid detection of Salmonella enteritidis by piezoelectric immunosensor. Microchem J 68:7
Babacan PP S, Letcher S, Rand A (2002) Piezoelectric flow injection analysis biosensor for the detection of salmonella typhimurium. Instit Food Technol 67:13
Wong YY, Ng SP, Ng MH et al (2002) Immunosensor for the differentiation and detection of salmonella species based on a quartz crystal microbalance. Biosens Bioelectron 17:676–684
Acknowledgments
This project was supported by the Food Science and Engineering the most important discipline of Zhejiang province (JYTSP20141062). The Talent training provincial superior paper funded project (1110JY1412001P). Postgraduate Scientific and Technological Innovation Project of Zhejiang Gongshang University (3100XJ1514146) and Plans for college students in Zhejiang Province science and technology innovation activities (acrobatic tender grass talent programme) project (1110JQ4212048G). Project supported by the fund of the National Natural Science Fund (30571623). Analysis and testing projects of Zhejiang public innovation platform (2015C37023).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 850 kb)
Rights and permissions
About this article
Cite this article
Fei, J., Dou, W. & Zhao, G. A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim Acta 182, 2267–2275 (2015). https://doi.org/10.1007/s00604-015-1573-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1573-x