Abstract
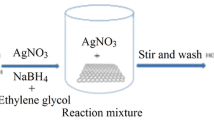
The article describes a facile method for the preparation of a conjugate composed of silver nanoparticles and graphene oxide (Ag@GO) via chemical reduction of silver precursors in the presence of graphene oxide (GO) while sonicating the solution. The Ag@GO was characterized by X-ray photoelectron spectroscopy, X-ray powder diffraction, and energy-dispersive X-ray spectroscopy. The nanocomposite undergoes a color change from yellow to colorless in presence of Hg(II), and this effect is based on the disappearance of the localized surface plasmon resonance absorption of the AgNPs due to the formation of silver-mercury amalgam. The presence of GO, on the other hand, prevents the agglomeration of the AgNPs and enhances the stability of the nanocomposite material in solution. Hence, the probe represents a viable optical probe for the determination of mercury(II) ions in that it can be used to visually detect Hg(II) concentrations as low as 100 μM. The instrumental LOD is 338 nM.

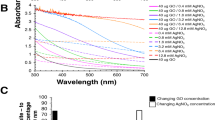
The mercury(II) ions interact with AgNP in Ag@GO nanocomposite and result in formation of AgHg amalgam. Therefore LSPR absorbance band of AgNPs starts to vanish. This mechanism can be used for developing a sensor for mercury(II) ions detection.






Similar content being viewed by others
References
Zhang L, Yu JC, Yip HY et al (2003) Ambient light reduction strategy to synthesize silver nanoparticles and silver-coated TiO2 with enhanced photocatalytic and bactericidal activities. Langmuir 19:10372–10380. doi:10.1021/la035330m
Jana NR, Sau TK, Pal T (1999) Growing small silver particle as redox catalyst growing small silver particle as redox catalyst. Grow small Met Part as redox. Catalysts 103:115–121. doi:10.1021/jp982731f
Tripathi GNR (2003) P-benzosemiquinone radical anion on silver nanoparticles in water. J Am Chem Soc 125:1178–1179. doi:10.1021/ja029049q
Ravi SS, Christena LR, SaiSubramanian N, Anthony SP (2013) Green synthesized silver nanoparticles for selective colorimetric sensing of Hg2+ in aqueous solution at wide pH range. Analyst 138:4370–4377. doi:10.1039/c3an00320e
Liang A, Liu Q, Wen G, Jiang Z (2012) The surface-plasmon-resonance effect of nanogold/silver and its analytical applications. TrAC Trends Anal Chem 37:32–47. doi:10.1016/j.trac.2012.03.015
Cobley CM, Skrabalak SE, Campbell DJ, Xia Y (2009) Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics 4:171–179. doi:10.1007/s11468-009-9088-0
Rivera VAG, Ferri FA EM Jr (2012) Localized surface plasmon resonances : noble metal nanoparticle interaction with rare-earth ions. Plasmon - Princ Appl. doi:10.5772/50753
Moores A, Goettmann F (2006) The plasmon band in noble metal nanoparticles: an introduction to theory and applications. New J Chem 30:1121. doi:10.1039/b604038c
Zangeneh Kamali K, Pandikumar A, Sivaraman G et al (2015) Silver@graphene oxide nanocomposite-based optical sensor platform for biomolecules. RSC Adv 5:17809–17816. doi:10.1039/C4RA11356J
Maduraiveeran G, Ramaraj R (2009) Potential sensing platform of silver nanoparticles embedded in functionalized silicate shell for nitroaromatic compounds. Anal Chem 81:7552–7560. doi:10.1021/ac900781d
Rameshkumar P, Viswanathan P, Ramaraj R (2014) Silicate sol-gel stabilized silver nanoparticles for sensor applications toward mercuric ions, hydrogen peroxide and nitrobenzene. Sensors Actuators B Chem 202:1070–1077. doi:10.1016/j.snb.2014.06.069
Golsheikh AM, Huang NM, Lim HN, Zakaria R (2014) One-pot sonochemical synthesis of reduced graphene oxide uniformly decorated with ultrafine silver nanoparticles for non-enzymatic detection of H 2 O 2 and optical detection of mercury ions. RSC Adv 4:36401. doi:10.1039/C4RA05998K
Langford NJ, Ferner RE (1999) Toxicity of mercury. J Hum Hypertens 13:651–656
Wang Y, Yang F, Yang X (2010) Colorimetric biosensing of mercury(II) ion using unmodified gold nanoparticle probes and thrombin-binding aptamer. Biosens Bioelectron 25:1994–1998. doi:10.1016/j.bios.2010.01.014
El-Safty SA, Shenashen MA (2012) Mercury-ion optical sensors. TrAC Trends Anal Chem 38:98–115. doi:10.1016/j.trac.2012.05.002
Farhadi K, Forough M, Molaei R et al (2012) Highly selective Hg2+ colorimetric sensor using green synthesized and unmodified silver nanoparticles. Sensors Actuators B Chem 161:880–885. doi:10.1016/j.snb.2011.11.052
Chemnasiri W, Hernandez FE (2012) Gold nanorod-based mercury sensor using functionalized glass substrates. Sensors Actuators B Chem 173:322–328. doi:10.1016/j.snb.2012.07.002
Chen L, Fu X, Lu W, Chen L (2013) Highly sensitive and selective colorimetric sensing of Hg2+ based on the morphology transition of silver nanoprisms. ACS Appl Mater Interfaces 5:284–290. doi:10.1021/am3020857
Chen X, Zu Y, Xie H et al (2011) Coordination of mercury(II) to gold nanoparticle associated nitrotriazole towards sensitive colorimetric detection of mercuric ion with a tunable dynamic range. Analyst 136:1690–1696. doi:10.1039/c0an00903b
Cho Y, Lee SS, Jung JH (2010) Recyclable fluorimetric and colorimetric mercury-specific sensor using porphyrin-functionalized Au@SiO2 core/shell nanoparticles. Analyst 135:1551–1555. doi:10.1039/c0an00137f
Vasimalai N, John SA (2012) Mercaptothiadiazole capped gold nanoparticles as fluorophore for the determination of nanomolar mercury(II) in aqueous solution in the presence of 50,000-fold major interferents. Analyst 137:3349–3354. doi:10.1039/c2an35190k
James JZ, Lucas D, Koshland CP (2013) Elemental mercury vapor interaction with individual gold nanorods. Analyst 138:2323–2328. doi:10.1039/c3an36841f
Jayabal S, Sathiyamurthi R, Ramaraj R (2014) Selective sensing of Hg2+ ions by optical and colorimetric methods using gold nanorods embedded in a functionalized silicate sol–gel matrix. J Mater Chem A 2:8918. doi:10.1039/c4ta01363h
Chen Y, Yao L, Deng Y et al (2015) Rapid and ultrasensitive colorimetric detection of mercury(II) by chemically initiated aggregation of gold nanoparticles. Microchim Acta. doi:10.1007/s00604-015-1538-0
Jena BK, Raj CR (2008) Gold nanoelectrode ensembles for the simultaneous electrochemical detection of ultratrace arsenic, mercury, and copper. Anal Chem 80:4836–4844. doi:10.1021/ac071064w
Alizadeh T, Ganjali MR, Zare M (2011) Application of an Hg2+ selective imprinted polymer as a new modifying agent for the preparation of a novel highly selective and sensitive electrochemical sensor for the determination of ultratrace mercury ions. Anal Chim Acta 689:52–59. doi:10.1016/j.aca.2011.01.036
Wu D, Zhang Q, Chu X et al (2010) Ultrasensitive electrochemical sensor for mercury (II) based on target-induced structure-switching DNA. Biosens Bioelectron 25:1025–1031. doi:10.1016/j.bios.2009.09.017
Deng L, Ouyang X, Jin J et al (2013) Exploiting the higher specificity of silver amalgamation: Selective detection of mercury(II) by forming Ag/Hg amalgam. Anal Chem 85:8594–8600. doi:10.1021/ac401408m
Lee H, Lee H-S, Reibenspies JH, Hancock RD (2012) Mechanism of “turn-on” fluorescent sensors for mercury(II) in solution and its implications for ligand design. Inorg Chem 51:10904–10915. doi:10.1021/ic301380w
Yan L, Chen Z, Zhang Z et al (2013) Fluorescent sensing of mercury(II) based on formation of catalytic gold nanoparticles. Analyst 138:4280–4283. doi:10.1039/c3an00725a
Bi N, Chen Y, Qi H et al (2012) Sensors and actuators B: chemical Spectrophotometric determination of mercury (II) ion using gold nanorod as probe. Sensors Actuators B Chem 166–167:766–771. doi:10.1016/j.snb.2012.03.068
Xu H, Wang Y, Huang X et al (2012) Hg2 + −mediated aggregation of gold nanoparticles for colorimetric screening of biothiols. Analyst 137:924. doi:10.1039/c2an15926k
Lou T, Chen Z, Wang Y, Chen L (2011) Blue-to-red colorimetric sensing strategy for Hg2+ and Ag+ via redox-regulated surface chemistry of gold nanoparticles. ACS Appl Mater Interfaces 3:1568–1573. doi:10.1021/am200130e
Wu C, Tse VM-L, Liu Z et al (2014) In-line microfluidic integration of photonic crystal fibre as a highly sensitive refractometer. Analyst. doi:10.1039/C4AN01361A
Xu LQ, Neoh K-G, Kang E-T, Fu GD (2013) Rhodamine derivative-modified filter papers for colorimetric and fluorescent detection of Hg2+ in aqueous media. J Mater Chem A 1:2526. doi:10.1039/c2ta01072k
Si SY, Si Y, Wang X et al (2014) Optimized colorimetric sensor strip for mercury(II) assay using hierarchical nanostructured conjugated polymers. J Mater Chem A. doi:10.1039/c3ta13867d
Slocik JM, Zabinski JS, Phillips DM, Naik RR (2008) Colorimetric response of peptide-functionalized gold nanoparticles to metal ions. Small 4:548–551
Ye T, He C, Qu Y et al (2012) A simple colorimetric device for rapid detection of Hg2+ in water. Analyst 137:4131–4134. doi:10.1039/c2an35422e
Wang G, Chen Z, Wang W et al (2011) Chemical redox-regulated mesoporous silica-coated gold nanorods for colorimetric probing of Hg2+ and S2 − .pdf. Analyst 136:174–178
Chen H-Q, Yuan F, Wang S-Z et al (2013) Near-infrared to near-infrared upconverting NaYF4:Yb3+, Tm3+ nanoparticles-aptamer-Au nanorods light resonance energy transfer system for the detection of mercuric(II) ions in solution. Analyst 138:2392–2397. doi:10.1039/c3an36921h
Anand GS, Gopalan AI, Kang S-W, Lee K-P (2013) Development of a surface plasmon assisted label-free calorimetric method for sensitive detection of mercury based on functionalized gold nanorods. J Anal At Spectrom 28:488. doi:10.1039/c3ja30300d
Huang NM, Lim HN, Chia CH et al (2011) Simple room-temperature preparation of high-yield large-area graphene oxide. Int J Nanomedicine 6:3443–3448. doi:10.2147/IJN.S26812
Jayabal S, Pandikumar A, Lim HN et al (2015) A gold nanorod-based localized surface plasmon resonance platform for the detection of environmentally toxic metal ions. Analyst 140:2540–2555. doi:10.1039/C4AN02330G
Sharma S, Sanpui P, Chattopadhyay A, Ghosh S (2012) Fabrication of antibacterial silver nanoparticle—sodium alginate–chitosan composite films. Rsc Adv 2:5837. doi:10.1039/c2ra00006g
Peter Amaladhas T, Sivagami S, Akkini Devi T et al (2012) Biogenic synthesis of silver nanoparticles by leaf extract of Cassia angustifolia. Adv Nat Sci Nanosci Nanotechnol 3:045006. doi:10.1088/2043-6262/3/4/045006
Paredes JI, Villar-Rodil S, Martínez-Alonso A, Tascón JMD (2008) Graphene oxide dispersions in organic solvents. Langmuir 24:10560–10564. doi:10.1021/la801744a
Ziaei E, Mehdinia A, Jabbari A (2014) A novel hierarchical nanobiocomposite of graphene oxide–magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal Chim Acta 850:49–56. doi:10.1016/j.aca.2014.08.048
Cui L, Guo X, Wei Q et al (2015) Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: sorption kinetic and uptake mechanism. J Colloid Interface Sci 439:112–120. doi:10.1016/j.jcis.2014.10.019
Morris T, Copeland H, McLinden E et al (2002) The effects of mercury adsorption on the optical response of size-selected gold and silver nanoparticles. Langmuir 18:7261–7264. doi:10.1021/la020229n
Liz-Marzán L (2004) Nanometals: formation and color. Mater Today 26–31
Radhakumary C, Sreenivasan K (2011) Gold nanoparticles generated through “green route” bind Hg2+ with a concomitant blue shift in plasmon absorption peak. Analyst 136:2959–2962. doi:10.1039/c1an15247e
Ramesh GV, Radhakrishnan TP (2011) A universal sensor for mercury (Hg, Hg I, Hg II) based on silver nanoparticle-embedded polymer thin film. ACS Appl Mater Interfaces 3:988–994. doi:10.1021/am200023w
Henglein A, Brancewicz C (1997) Absorption spectra and reactions of colloidal bimetallic nanoparticles containing mercury. Chem Mater 9:2164–2167
Bera RK, Das AK, Raj CR (2010) Enzyme-cofactor-assisted photochemical synthesis of Ag nanostructures and shape-dependent optical sensing of Hg(II) ions. Chem Mater 22:4505–4511. doi:10.1021/cm1013762
Li W, Guo Y, McGill K, Zhang P (2010) A facile synthesis of Ag nanoparticles for mercury ion detection with high sensitivity and selectivity. New J Chem 34:1148. doi:10.1039/b9nj00630c
Henglein A, Brancewicz C (1997) Absorption spectra and reactions of colloidal bimetallic nanoparticles containing mercury. Chem Mater 4756:2164–2167
Zumdahl SS, Zumdahl SA (2007) Chemistry, 7th edn. Massachusetts Houghton Miffle Company, New Jersey
Petrucci RH, Harwood WS, Herring GE, Madura J (2007) General chemistry: principles and modern applications, 9th edn. Pearson Education, Upper Saddle River
Sohn JH, Pham LQ, Kang HS et al (2010) Preparation of conducting silver paste with Ag nanoparticles prepared by e-beam irradiation. Radiat Phys Chem 79:1149–1153. doi:10.1016/j.radphyschem.2010.06.005
Alexander V, Naumkin AK-V, Gaarenstroom SW, Powell CJ (2012) NIST X-ray photoelectron spectroscopy database. In: Natl Inst Stand Technol Gaithersbg. http://srdata.nist.gov/xps/
Vasimalai N, John SA (2013) Micromolar Hg(ii) induced the morphology of gold nanoparticles: a novel luminescent sensor for femtomolar Hg(ii) using triazole capped gold nanoparticles as a fluorophore. J Mater Chem A 1:4475. doi:10.1039/c3ta00003f
Henglein A (1998) Colloidal silver nanoparticles : photochemical preparation and interaction with O 2, CCl 4, and some. Chem Mater 2:444–450
Acknowledgments
This work was supported by the High Impact Research Grant from the Ministry of Higher Education of Malaysia (UM.C/625/1/HIR/MOHE/05) and University of Malaysia Research Grant, UMRG Programme (RP007C/13AFR).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 447 kb)
Rights and permissions
About this article
Cite this article
Zangeneh Kamali, K., Pandikumar, A., Jayabal, S. et al. Amalgamation based optical and colorimetric sensing of mercury(II) ions with silver@graphene oxide nanocomposite materials. Microchim Acta 183, 369–377 (2016). https://doi.org/10.1007/s00604-015-1658-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1658-6