Abstract
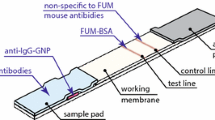
The authors describe the preparation of desert rose-like gold nanoparticles (DR-GNPs) with a plasmon resonance band at 620 nm which gives them a blue color. They have a hydrodynamic diameter of ∼72 nm and were prepared by a seeding growth approach. The DR-GNPs were characterized by UV-vis spectroscopy, transmission electron microscopy and dynamic light scattering. These nonspherical GNPs were used as a label for the antibody in an immunochromatographic strip test (ICST). Despite their particular shape and the higher surface area compared to spherical gold nanoparticles, the DR-GNPs are useful blue labels for the GNP-based strip test. A multicolor ICST for aflatoxin B1 and fumonisins is described that employs both blue DR-GNPs and red spherical GNPs. It allows for simultaneous rapid determination of the two mycotoxins in maize flour, with visual cut-off levels of 2 μg⋅kg-1 for aflatoxin B1 and of 1000 μg⋅kg-1 for fumonisins.

Blue desert rose-like gold nanoparticles (DR-GNPs) were synthesized, characterized and applied as label for the ImmunoChromatographic Strip Test (ICST) technique, in which red spherical GNPs (s-GNPs) are usually employed. The combined use of the blue DR-GNP and red s-GNPs allowed developing of an intuitive multicolor ICST for the simultaneous detection of aflatoxin B1 and fumonisins in maize flour.






Similar content being viewed by others
References
Prakash J, Pivin JC, Swart HC (2015) Noble metal nanoparticles embedding into polymeric materials: from fundamentals to applications. Adv Coll Interface Sci 226:187–202. doi:10.1016/j.cis.2015.10.010
Sardar R, Funston AM, Mulvaney P, Murray RW (2009) Gold nanoparticles: past, present, and future. Langmuir 25:13840–13851. doi:10.1021/la9019475
Liu X, Wang Y, Chen P, Wang Y, Zhang J, Aili D, Liedberg B (2014) Biofunctionalized gold nanoparticles for colorimetric sensing of Botulinum neurotoxin a light chain. Anal Chem 86:2345–2352. doi:10.1021/ac402626g
Gao Y, Li X, Li Y, Li T, Zhao Y, Wu A (2014) A simple visual and highly selective colorimetric detection of Hg2+ based on gold nanoparticles modified by 8-hydroxyquinolines and oxalates. Chem Commun 50:6447–6450. doi:10.1039/c4cc00069b
Li YS, Zhou Y, Meng XY, Zhang YY, Song F, Lu SY, Ren HLHP, Liu ZS, Zhang JH (2014) Gold nanoparticle aggregation-based colorimetric assay for β–casein detection in bovine milk samples. Food Chem 162:22–26. doi:10.1016/j.foodchem.2014.04.049
Omidfar K, Khorsand F, Azizi MD (2013) New analytical applications of gold nanoparticles as label in antibody based sensors. Biosens Bioelectron 43:336–347. doi:10.1016/j.bios.2012.12.045
Chun P (2009) Colloidal gold and other labels for lateral flow immunoassays. In: Wong RC, Tse HY (eds) Lateral flow immunoassay, 1st edn. Humana Press, New York, pp 75–93
Mak WC, Beni V, Turner APF (2016) Lateral-flow technology: from visual to instrumental. TrAC Trends Anal Chem 79:297–305. doi:10.1016/j.trac.2015.10.017
Han S, Zhou T, Yin B, He P (2016) A sensitive and semi-quantitative method for determination of multidrug residues in animal body fluids using multiplex dipstick immunoassay. Anal Chim Acta 927:64–71. doi:10.1016/j.aca.2016.05.004
Zhang L, Huang Y, Wang J, Rong Y, Lai W, Zhang J, Chen T (2015) Hierarchical flowerlike gold nanoparticles labeled immunochromatography test strip for highly sensitive detection of Escherichia coli O157:H7. Langmuir 31:5537–5554
Ji Y, Ren M, Li Y, Huang Z, Shu M, Yang H, Xiong Y, Xu Y (2015) Detection of aflatoxin B1 with immunochromatographic test strips: enhanced signal sensitivity using gold nanoflowers. Talanta 142:206–212. doi:10.1016/j.talanta.2015.04.048
Wiriyachaiporn N, Maneeprakorn W, Apiwat C, Dharakul T (2015) Dual-layered and double-targeted nanogold based lateral flow immunoassay for influenza virus. Microchim Acta 182:85–93
Man Y, Lv X, Iqbal J, Peng G, Song D, Zhang C, Deng Y (2015) Microchip based and immunochromatographic strip assays for the visual detection of interleukin-6 and of tumor necrosis factor α using gold nanoparticles as labels. Microchim Acta 182:597–604
Luan Y, Chen J, Xie G, Li C, Ping H, Ma Z, Lu A (2015) Visual and microplate detection of aflatoxin B2 based on NaCl-induced aggregation of aptamer-modified gold nanoparticles. Microchim Acta 182:995–1001
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182:571–578
Brown MC (2009) Antibodies: key to a robust lateral flow immunoassay. In: Wong RC, Tse HY (eds) Lateral flow immunoassay, 1st edn. Humana Press, New York, pp 59–74
O'Farrell B (2015) Lateral flow Technology for Field-Based Applications-Basics and Advanced Developments. Topics Compan Anim Med 30:139–147. doi:10.1053/j.tcam.2015.12.003
Anfossi L, Giovannoli C, Baggiani C (2016) Mycotoxin detection. Curr Opin Biotechnol 37:120–126. doi:10.1016/j.copbio.2015.11.005
Taranova NA, Berlina AN, Zherdev AV, Dzantiev BB (2014) 'Traffic light' immunochromatographic test based on multicolor quantum dots for the simultaneous detection of several antibiotics in milk. Biosens Bioelectron 63:255–261. doi:10.1016/j.bios.2014.07.049
Foubert A, Beloglazova NV, Gordienko A, Tessier MD, Drijvers E, Hens Z, De Saeger S (2016) Development of a rainbow lateral flow immunoassay for the simultaneous detection of four mycotoxins. J Agric Food Chem. doi:10.1021/acs.jafc.6b04157
Li J, Wu J, Zhang X, Liu Y, Zhou D, Sun H, Zhang H, Yang B (2011) Controllable synthesis of stable urchin-like gold nanoparticles using hydroquinone to tune the reactivity of gold chloride. J Phys Chem C 115:3630–3637. doi:10.1021/jp1119074
Zhao L, Ji X, Sun X, Li J, Yang W, Peng X (2009) Formation and stability of gold nanoflowers by the seeding approach: the effect of Intraparticle ripening. J Phys Chem C 113:16645–16651. doi:10.1021/jp9058406
Yen CW, de Puig H, Tam JO, Gómez-Márquez J, Bosch I, Hamad-Schifferli K, Gehrke L (2015) Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing dengue, yellow fever, and Ebola viruses. Lab Chip 15:1638–1641. doi:10.1039/c5lc00055f
International Agency for Research on Cancer, IARC (2002) Evaluation of carcinogenic risks in humans. Some traditional herbal medicines, some mycotoxins, naphtalene and styrene, vol. 82. IARC Press, Lyon, pp 171–274
Commission Regulation (EU) No 165/2010 (2010) Off J Eur Comm L50:8–12
Di Nardo F, Anfossi L, Giovannoli C, Passini C, Goftmann VV, Goryacheva IY, Baggiani C (2016) A fluorescent immunochromatographic strip test using quantum dots for fumonisins detection. Talanta 150:463–468. doi:10.1016/j.talanta.2015.12.072
Marasas WFO (2001) Discovery and occurrence of the fumonisins: a historical perspective. Environ Health Perspect 109:239–243. doi:10.2307/3435014
International Agency for Research on Cancer, IARC (2002) Monographs on the evaluation of the carcinogenic risk of chemicals to humans. Some traditional herbal medicines, some mycotoxins, naphtalene and styrene, vol. 82. IARC Press, Lyon, pp 301–366
Commission Regulation (EC) No. 1126/2007 (2007) Off J Eur Comm L255:14–17
Goryacheva IY, De Saeger S, Eremin SA, Van Peteghem C (2007) Immunochemical methods for rapid mycotoxin detection: evolution from single to multiple analyte screening: a review. Food Addi ContamA 24:1169–1183. doi:10.1080/02652030701557179
Krska R, Schubert-Ullrich P, Molinelli A, Sulyok M, MacDonald S, Crews C (2008) Mycotoxin analysis: an update. Food AddContam A 25:152–163. doi:10.1080/02652030701765723
Anfossi L, Calderara M, Baggiani C, Giovannoli C, Arletti E, Giraudi G (2010) Development and application of a quantitative lateral flow immunoassay for fumonisins in maize. Anal Chim Acta 682:104–109. doi:10.1016/j.aca.2010.09.045
Khlebstov NG (2008) Determination of size and concentration of gold nanoparticles from extinction spectra. Anal Chem 80:6620–6625. doi:10.1021/ac800834n
Jazayeri MH, Amani H, Pourfatollah A, Pazoki-Toroudi H, Sedighimoghaddam B (2016) Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sens Biosensing Res 9:17–22. doi:10.1016/j.sbsr.2016.04.002
Daohong Zhang D, Li P, Zhang Q, Zhang W (2011) Ultrasensitive nanogold probe-based immunochromatographic assay for simultaneous detection of total aflatoxins in peanuts. Biosens Bioelectron 26:2877–2882. doi:10.1016/j.bios.2010.11.031
Oliver C (2010) Conjugation of colloidal gold to proteins. Meth Mol Biol 588:369–373. doi:10.1007/978-1-59745-324-0_39
Majdinasab M, Sheikh-Zeinoddin M, Soleimanian-Zad S, Li P, Zhang Q, Li X, Tang X (2015) Ultrasensitive and quantitative gold nanoparticle-based immunochromatographic assay for detection of ochratoxin a in agro-products. J Chromatogr B 974:147–154. doi:10.1016/j.jchromb.2014.10.034
Di Nardo F, Anfossi L, Ozella L, Saccani A, Giovannoli C, Spano G, Baggiani C (2016) Validation of a qualitative immunochromatographic test for the noninvasive assessment of stress in dogs. J Chromatogr B 1028:192–198. doi:10.1016/j.jchromb.2016.06.019
Li Z, Wang Y, Wang J, Tang Z, Pounds JG, Lin Y (2010) Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem 82:7008–7014. doi:10.1021/ac101405a
Tatona K, Johnsona D, Guirea P, Langeb E, Tondra M (2009) Lateral flow immunoassay using magnetoresistive sensors. J Magn Magn Mater 321:1679–1682
Du D, Wang J, Wang L, Lu D, Lin Y (2012) Integrated lateral flow test strip with electrochemical sensor for quantification of phosphorylated cholinesterase: biomarker of exposure to Organophosphorus agents. Anal Chem 84:1380–1385
Parolo C, de la Escosura-Muñiza A, Merkoçi A (2013) Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens Bioelectron 40:412–416
Rivas L, de la Escosura-Muñiz A, Serrano L, Altet L, Francino O, Sánchez A, Merkoçi A (2015) Triple lines gold nanoparticle-based lateral flow assay for enhanced and simultaneous detection of Leishmania DNA and endogenous control. Nano Res 8:3704–3714
Quesada-González D, Merkoçi A (2015) Nanoparticle-based lateral flow biosensors. Biosens Bioelectron 73:47–63
Sajid M, Kawde A-N, Daud M (2015) Designs, formats and applications of lateral flow assay: a literature review. J Saudi Chem Soc 19:689–705
Chauhan R, Singh J, Sachdev T, Basu T, Malhotra BD (2016) Recent advances in mycotoxins detection. Biosens Bioelectron 81:532–545. doi:10.1016/j.bios.2016.03.004
Maragos CM (2016) Multiplexed biosensors for mycotoxins. J AOAC Int 99:849–860
Zangheri M, Di Nardo F, Anfossi L, Giovannoli C, Baggiani C, Roda A, Mirasoli M (2015) A multiplex chemiluminescent biosensor for type B-fumonisins and aflatoxin B1 quantitative detection in maize flour. Analyst 140:358–365. doi:10.1039/c4an01613k
Acknowledgements
Authors are grateful to Dr. M. Manzoli and Prof. V. Maurino for the fruitful and helpful discussion related to the TEM and DLS measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 2470 kb)
Rights and permissions
About this article
Cite this article
Di Nardo, F., Baggiani, C., Giovannoli, C. et al. Multicolor immunochromatographic strip test based on gold nanoparticles for the determination of aflatoxin B1 and fumonisins. Microchim Acta 184, 1295–1304 (2017). https://doi.org/10.1007/s00604-017-2121-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2121-7