Abstract
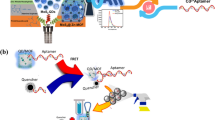
The authors have designed a DNAzyme where graphene oxide (GO) interacts with the ssDNA stem loop region. The DNAzyme strand and substrate strand are hybridized and bind to the surface of GO which act as a signal reporter, while GO act as a strong quencher. The presence of Pb(II) ion disturbs the GO-DNAzyme complex and causes internal cleavage of the DNAzyme complex. On addition of Thioflavin T (ThT) as a quadruplex inducer, fluorescence intensity (best measured at excitation/emission peaks of 425/490 nm) is strongly enhanced. Subsequent addition of Hg(II) to ThT/G-quadruplex complex decreases fluorescence because the G-quadruplex is unwinding to form a T-Hg(II)-T dsDNA system. Therefore, the change in fluorescence intensity of ThT is directly correlated to the concentration of Pb(II) and Hg(II). As a result, the assay is highly selective and sensitive. The limits of detection are 96 pM for Pb(II) and 356 pM for Hg(II). Moreover, the method was applied to the detection of the two ions in spiked real samples and gave satisfactory results.

A label free sensitive and selective “on-off” fluorescent assay for detection of Pb(II) and Hg(II) based on graphene oxide –DNAzyme complex with fluorogenic dye thioflavin T. The limits of detection are 96 pM (Pb2+) and 356 pM (Hg2+).





Similar content being viewed by others
References
Needleman HL (2004) Lead poisoning. Annu Rev Med 55:209–222
Godwin HA (2001) The biological chemistry of lead. Curr Opin Chem Biol 5:223–227
Goldstein G (1992) Developmental neurobiology of lead toxicity. In: Needleman H (ed) Human lead exposure. CRC Press, Taylor & Francis, London, pp 125–135
Nodan EM, Lippard SJ (2008) Small- molecule fluorescence sensors for investigating zinc metallonuerochemistry. Chem Rev 42(1):193–203
Zarlaida F, Adlim M (2017) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review. Microchim Acta 184:45–58
Ghaedi M, Reza Fathi M, ShoKrollahi M, Shajarat F (2006) Highly selective and sensitive preconcentration of mercury ion and determination by cold vapour atomic absorption spectroscopy. Anal Lett 39:1171–1185
Puk R, Weber JH (1994) Determination of mercury(II), monomethylmercury cation, dimethylmercury and diethylmercury and hydride generation, cryogenic trapping and atomic adsorption spectrometric detection. Anal Chim Acta 292:175–183
Jia X, Han Y, Liu X, Duran T, Chen H (2011) Specification of mercury in liquid-liquid micro extraction combined with high performance liquid chromatography and inductively coupled plasma mass spectrometry. Spectrochim Acta Part B 66:88–92
Beqa L, Singh AK, Khan SA, Senapati D, Arumugam SR, Ray PC (2011) Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the for the selective detection of Pb(II) from paints, plastics and water samples. ACS Appl Mater Interfaces 3:668–673
Liu Y, Li X, Wang G, Tang W (2013) A highly sensitive and selective optical sensor for Pb2+ by using conjugated polymers and label-free oligonucleotides. Biosens Bioelectron 39:231–235
Lan T, Furuya K, Lu Y (2010) A highly selective lead sensor based on a lead DNAzyme. Chem Commun 46:3896–3898
Liu J, Lu Y (2007) A DNAzyme catalytic beacon sensor for paramagnetic Cu2+ ions in aqueous solution with high sensitivity and selectivity. J Am Chem Soc 129:9838–9839
Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y (2007) A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and million fold selectivity. Proc Natl Acad Sci U. S. a 104:2056
Li J, Zhang W, Kwon AH, Lu Y (2000) In vitro selection and characterization of a highly efficient Zn(II) dependent RNA-cleaving deoxyribozyme. Nucleic Acids Res 28:481–488
Wang L, Jin Y, Deng J, Chen GZ (2011) Gold nanorods - based FRET assay for sensitive detection Pb2+ using 8-17 DNAzyme. Analyst 136:5169–5174
Liu Z, Robinson JT, Sun XM, Dai J (2008) PEGylated nano-graphene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc 130:10876–10877
Toda K, Furue R, Hayami S (2015) Recent progress in applications of graphene oxide for gas sensing: a review. Anal Chim Acta 878:43–53
Park H, Hwang SJ, Kim K (2012) An electrochemical detection of Hg2+ion using graphene oxide as an electrochemically active indicator. Electrochem Commun 24:100–103
Wang H, Yang RH, Yang L, Tan WH (2009) Nucleic acid conjugated nonmaterial’s for enhanced molecular recognition. ACS Nano 3:2451–2460
Manohar S, Mantz AR, Bancroft KE, Hui CY, Jagota A, Vezenov DZ (2008) Peeling single stranded DNA from graphite surface to determine oligonucleotide binding energy by force spectroscopy. Nano Lett 8(4365):4372
PJJ H, Liu J (2012) Molecular beacon lighting up on graphene oxide. Anal Chem 84:4192–4198
Liu B, Sun Z, Zhang X, Liu J (2013) Mechanisms of sensing on graphene oxide. Anal Chem 85(16):7987–7993
Mohanty J, Barooah N, Dhamodharan V, Harikrishna V, Pradeepkumar PI, Bhasikuttan AC (2013) Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA. J Am Chem Soc 135(1):367–376
Ge J, Li XP, Jiang JH, Yu RQ (2014) A highly sensitive label-free sensor for mercury ion (Hg2+) by inhibiting thioflavin T as DNA G-quadruplex fluorescent inducer. Talanta 122:85–90
Sigel RKO, Sigel H (2000) A stability concept for metal ion coordination to single-stranded nucleic acids affinities of individual sites. Acc Chem 39:5985–5993
Pang S, Liu S, Su X (2015) An ultrasensitive sensing strategy for the detection of lead ions based on the intermolecular G-quadruplex and graphene oxide. Sensors Actuators B 208:415–420
Bai Y, Zhao L, Chen Z, Wang H, Feng F (2014) A label-free fluorescent sensor for Pb2+ based on G-quadruplex and graphene oxide. Anal Methods 6:8120–8123
Yan M, Zhu C, Huang Y, Yan J, Chen A (2017) Ultrasensitive detection of lead(II) using a turn-on probe based on the use of an aptamer and a water- soluble fluorescent perylene probe. Microchim Acta 184(7):2439–2444
Fu T, Ren S, Gong L, Meng H, Cui L, MeiKong R, Zhang XB, Tan W (2016) A label-free DNAzyme fluorescence biosensor for amplified detection of Pb2+ based on cleavage-induced G-quadruplex formation. Talanta 147:302–306
Li X, Wang G, Ding X, Che Y, Gou Y, Lu Y (2013) A “turn-on” fluorescent sensor for detection Pb2+ based on graphene oxide and G-qudruplex DNA. Phys Chem Chem Phys 15:12800
Zhao XH, Kong RM, Zhang XB, Meng HM, Tan WN, Shen GL, Yu RQ (2011) Graphene-DNAzyme based biosensor for amplified fluorescence “turn-on” detection of Pb2+ with a high selectivity. Anal Chem 83:5062–5066
Zhang JR, Huang WT, Xie WY, Wen T, Luo HQ, Li NB (2012) Highly sensitive ,selective, and rapid fluorescence hg(II) ion sensor based on DNA duplexes of poly(dT) and graphene oxide. Analyst 137:3300–3305
Li M, Zhou X, Ding W, Guo S, Wu N (2013) Graphene quantum dots and graphene oxide based biosensor detection of cancer biomarker and heavy metals. Biosens Bioelectron 41:889–893
Tianyu H, Xu Y, Weidan N, Xingguang S (2016) Aptamer-based aggregation assay for mercury(II) using gold nanoparticles and fluorescent CdTe quantum dots. Microchim Acta 183(7):2131–2137
Teha HB, Wub H, Zuoa X, Li SFY (2014) Detection of hg(II) ion using molecular beacon-based fluorescent sensor with high sensitivity and tunable dynamic range. Sensors Actuators B 195:623–629
Li Y, Liu N, Liu H, Wang Y, Hao Y, Ma X, Li X, Huo Y, Lu J, Tang S, Wang C, Zhang Y, Gao Z (2017) A novel label-free fluorescence assay for one-step sensitive detection of Hg2+ in environmental drinking water. Sci Rep 7:45974
Acknowledgements
Author acknowledges the financial support from the Department of Chemistry, SRM University, Tamil Nadu - 603 203, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 872 kb)
Rights and permissions
About this article
Cite this article
Ravikumar, A., Panneerselvam, P. & Radhakrishnan, K. Fluorometric determination of lead(II) and mercury(II) based on their interaction with a complex formed between graphene oxide and a DNAzyme. Microchim Acta 185, 2 (2018). https://doi.org/10.1007/s00604-017-2585-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2585-5