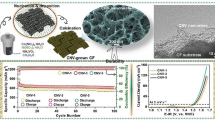
Abstract
Core-shell structured particles were prepared from carbonized zeolitic imidazolate frameworks (ZIFs) and reduced graphene oxide (rGO). The particles possess a nitrogen content of up to 10.6%. The loss of nitrogen from the ZIF is avoided by utilizing the reduction and agglomeration of graphene oxide with suitable size (>2 μm) during pyrolysis. The resulting carbonized ZIF@rGO particles were deposited on a glassy carbon electrode to give an amperometric sensor for H2O2, typically operated at a voltage of −0.4 V (vs. Ag/AgCl). The sensor has a wide detection range (from 5 × 10−6 to 2 × 10−2 M), a 3.3 μM (S/N = 3) detection limit and a 0.272 μA·μM−1·cm−2 sensitivity, much higher than that of directly carbonized ZIFs. The sensor material was also deposited on a screen-printed electrode to explore the possibility of application.

Nitrogen doped carbon (NC) derived from carbonized zeolitic imidazolate frameworks is limited because of low nitrogen content. Here, nitrogen-rich NC@reduced graphene oxide (rGO) core-shell structured particles are described. The NC@rGO particles show distinctly better H2O2 detection performance than NC.






Similar content being viewed by others
References
Jiang J, Oberdörster G, Elder A, Gelein R, Mercer P, Biswas P (2008) Does nanoparticle activity depend upon size and crystal phase? Nanotoxicology 2(1):33–42
Sun Y, Luo M, Qin Y, Zhu S, Li Y, Xu N, Meng X, Ren Q, Wang L, Guo S (2017) Atomic-thick PtNi nanowires assembled on graphene for high-sensitivity extra-cellular hydrogen peroxide sensors. ACS Appl Mater Inter 9(40):34715–34721
Duan N, Jiang L, Xu F, Zhang G (2018) A non-contact original-state online real-time monitoring method for complex liquids in industrial processes. Engineering 4:392–397
Wu X, Li M, Li Z, Lv L, Zhang Y, Li C (2017) Amyloid-graphene oxide as immobilization platform of au nanocatalysts and enzymes for improved glucose-sensing activity. J Colloid Interf Sci 490:336–342
Roberts JG, Voinov MA, Schmidt AC, Smirnova TI, Sombers LA (2017) The hydroxyl radical is a critical intermediate in the voltammetric detection of hydrogen peroxide. J Am Chem Soc 138(8):2516–2519
Chen J, Ji X, He Z (2017) Smart composite reagent composed of double-stranded DNA-templated copper nanoparticle and SYBR green I for hydrogen peroxide related biosensing. Anal Chem 89(7):3988–3995
Mei H, Wu W, Yu B, Wu H, Wang S, Xia Q (2016) Nonenzymatic electrochemical sensor based on Fe@Pt core–shell nanoparticles for hydrogen peroxide, glucose and formaldehyde. Sensor Actuat B Chem 223:68–75
Zhang Y, Huang B, Yu F, Yuan Q, Gu M, Ji J, Zhang Y, Li Y (2018) 3D nitrogen-doped graphite foam@Prussian blue: an electrochemical sensing platform for highly sensitive determination of H2O2 and glucose. Microchim Acta 185(2):86. https://doi.org/10.1007/s00604-017-2631-3
Dilpazir S, He H, Li Z, Wang M, Lu P, Liu R, Xie Z, Gao D, Zhang G (2018) Cobalt single atoms immobilized n-doped carbon nanotubes for enhanced bifunctional catalysis toward oxygen reduction and oxygen evolution reactions. ACS Appl Energ Mater 1(7):3283–3291
Li Z, Yang L, Cao H, Chang Y, Tang K, Cao Z, Chang J, Cao Y, Wang W, Gao M, Liu C, Liu D, Zhao H, Zhang Y, Li M (2017) Carbon materials derived from chitosan/cellulose cryogel-supported zeolite imidazole frameworks for potential supercapacitor application. Carbohyd Polym 175:223–230
Shen K, Zhang L, Chen X, Liu L, Zhang D, Han Y, Chen J, Long J, Luque R, Li Y, Chen B (2018) Ordered macro-microporous metal-organic framework single crystals. Science 359(6372):206–210
Zehui Li, Hongyan He, Hongbin Cao, Shaoming Sun, Wenlin Diao, Denglei Gao, Peilong Lu, Shuangshuang Zhang, Zhuang Guo, Mingjie Li, Rongji Liu, Dunhao Ren, Chenming Liu, Yi Zhang, Zheng Yang, Jingkun Jiang, Guangjin Zhang (2019) Atomic Co/Ni dual sites and Co/Ni alloy nanoparticles in N-doped porous Janus-like carbon frameworks for bifunctional oxygen electrocatalysis. Appl Catal B Environ 240:112–121
Thomas M, Illathvalappil R, Kurungot S, Nair BN, Mohamed AA, Anilkumar GM, Yamaguchi T, Hareesh US (2016) Graphene oxide sheathed ZIF-8 microcrystals: engineered precursors of nitrogen-doped porous carbon for efficient oxygen reduction reaction (ORR) electrocatalysis. ACS Appl Mater Inter 8(43):29373–29382
Wei J, Hu Y, Liang Y, Kong B, Zhang J, Song J, Bao Q, Simon GP, Jiang SP, Wang H (2015) Electrocatalysts: nitrogen-doped nanoporous carbon/graphene nano-sandwiches: synthesis and application for efficient oxygen reduction. Adv Funct Mater 25(36):5876–5876
Hao Y, Xu Y, Liu J, Sun X (2017) Nickel–cobalt oxides supported on co/N decorated graphene as an excellent bifunctional oxygen catalyst. J Mater Chem A 5(11):5594–5600
Wen J, Li M, Xiao J, Liu C, Li Z, Xie Y, Ning P, Cao H, Zhang Y (2016) Novel oxidative cutting graphene oxide to graphene quantum dots for electrochemical sensing application. Mater Today Commun 8:127–133
Wang X, Zhi L, Müllen K (2008) Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett 8(1):323–327
Zhong HX, Wang J, Zhang YW, Xu WL, Xing W, Xu D, Zhang YF, Zhang XB (2014) ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew Chem 53(51):14235–14239
Li Z, Wang W, Cao H, Zhang Q, Zhou X, Wang D, Wang Y, Zhang S, Zhang G, Liu C (2017) Boron doped ZIF-67@graphene derived carbon electrocatalyst for highly efficient enzyme-free hydrogen peroxide biosensor. Adv Mater Technol 2(12):1700224
Zhong S, Zhan C, Cao D (2015) Zeolitic imidazolate framework-derived nitrogen-doped porous carbons as high performance supercapacitor electrode materials. Carbon 85:51–59
Li M, Li Z, Liu C, Chang Y, Wen J, Zhao H, Cao H, Zhang Y, Liu D (2016) Amino-modification and successive electrochemical reduction of graphene oxide for highly sensitive electrochemical detection of trace Pb2+. Carbon 109(11):479–486
Hou Y, Wen Z, Cui S, Ci S, Mao S, Chen J (2015) An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv Funct Mater 25(6):872–882
Guo D, Shibuya R, Akiba C, Saji S, Kondo T, Nakamura J (2016) Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351(6271):361–365
Michopoulos A, Kouloumpis A, Gournis D, Prodromidis MI (2014) Performance of layer-by-layer deposited low dimensional building blocks of graphene-prussian blue onto graphite screen-printed electrodes as sensors for hydrogen peroxide. Electrochim Acta 146:477–484
Gatselou VA, Giokas DL, Vlessidis AG, Prodromidis MI (2015) Rhodium nanoparticle-modified screen-printed graphite electrodes for the determination of hydrogen peroxide in tea extracts in the presence of oxygen. Talanta 134:482–487
Sun Y, Luo M, Meng X, Xiang J, Wang L, Ren Q, Guo S (2017) Graphene/intermetallic PtPb nanoplates composites for boosting electrochemical detection of H2O2 released from cells. Anal Chem 89(6):3761–3767
Yusoff N, Rameshkumar P, Mehmood MS, Pandikumar A, Lee HW, Huang NM (2017) Ternary nanohybrid of reduced graphene oxide-nafion@ silver nanoparticles for boosting the sensor performance in non-enzymatic amperometric detection of hydrogen peroxide. Biosens Bioelectron 87:1020–1028
Jahanbakhshi M (2018) Myoglobin immobilized on mesoporous carbon foam in a hydrogel (selep) dispersant for voltammetric sensing of hydrogen peroxide. Microchim Acta 185:121. https://doi.org/10.1007/s00604-017-2654-9
Lyu YP, Wu YS, Wang TP, Lee CL, Chung MY, Lo CT (2018) Hydrothermal and plasma nitrided electrospun carbon nanofibers for amperometric sensing of hydrogen peroxide. Microchim Acta 185:371. https://doi.org/10.1007/s00604-018-2915-2
Liu J, Yang C, Shang Y, Zhang P, Liu J, Zheng J (2018) Preparation of a nanocomposite material consisting of cuprous oxide, polyaniline and reduced graphene oxide, and its application to the electrochemical determination of hydrogen peroxide. Microchim Acta 185(3):172. https://doi.org/10.1007/s00604-018-2717-6
Fu L, Wang A, Lai G, Lin CT, Yu J, Yu A, Liu Z, Xie K, Su W (2018) A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol. Microchim Acta 185(2):87. https://doi.org/10.1007/s00604-017-2646-9
Wang MQ, Zhang Y, Bao SJ, Yu YN, Ye C (2016) Ni(II)-based metal-organic framework anchored on carbon nanotubes for highly sensitive non-enzymatic hydrogen peroxide sensing. Electrochim Acta 190:365–370
Cheng C, Zhang C, Gao X, Zhuang Z, Du C, Chen W (2018) 3D network and 2D paper of reduced graphene oxide/Cu2O composite for electrochemical sensing of hydrogen peroxide. Anal Chem 90(3):1983–1991
Li Z, Jiang Y, Liu C, Wang Z, Cao Z, Yuan Y, Li M, Wang Y, Fang D, Guo Z, Wang D, Zhang G, Jiang J (2018) Emerging investigator series: dispersed transition metal on nitrogen doped carbon nanoframework for environmental hydrogen peroxide detection. Environ Sci: Nano 5:1834–1843
Zhou M, Zhai Y, Dong S (2009) Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal Chem 81(14):5603–5613
Bas SZ, Cummins C, Borah D, Ozmen M, Morris MA (2018) Electrochemical sensing of hydrogen peroxide using block copolymer templated iron oxide nanopatterns. Anal Chem 90(2):1122–1128
Acknowledgements
Financial supports from the National Key R&D Program of China (2016YFC0200102), the National Natural Science Foundation of China (91643201 & 51608509 & 91545125 & U1662121) and Tsinghua Qingfeng Scholarship (THQF2018-16) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1740 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Jiang, Y., Wang, Z. et al. Nitrogen-rich core-shell structured particles consisting of carbonized zeolitic imidazolate frameworks and reduced graphene oxide for amperometric determination of hydrogen peroxide. Microchim Acta 185, 501 (2018). https://doi.org/10.1007/s00604-018-3032-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3032-y