Abstract
A method is described for sensitive and selective detection of iodine by using a paper strip modified with silver triangular nanoplates (AgTNPs). It is based on the extraction of iodine from a solution into a flow of air via dynamic gas extraction and transferring it through a reactive paper modified with AgTNPs. The interaction of AgTNPs with iodine results in a color change from blue to white. This can be visually detected and monitored by digital colorimetry. The dynamic gas extraction is highly selective for volatile compounds so that a sample pretreatment is minimal. Due to the sensitivity of AgTNPs for iodine, the limit of its detection is as low as 7 μg L−1, and the analytical range is of 20–200 μg L−1. The method also was applied in a new approach for determination of organic compounds that can interact with iodine. The organic compound is exposed to an excess of iodine, and this is followed by detection of residual iodine as described above. The method was applied to the determination of ascorbic acid, caffeine and the drug metamizole.

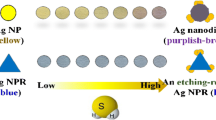
Schematic representation of a procedure of organic iodine-interacting compounds (Org.) determination. It is based on their iodination followed by gas extraction of the residual iodine, its interaction with silver triangular nanoplates and colorimetric detection with a scanner.







Similar content being viewed by others
References
Priyadarshini E, Pradhan N (2017) Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sensors Actuators B Chem 238:888–902
Fang C, Dharmarajan R, Megharaj M, Naidu R (2017) Gold nanoparticle-based optical sensors for selected anionic contaminants. Trends Anal Chem 86:143–154
Lim M-C, Kim Y-R (2016) Analytical applications of nanomaterials in monitoring biological and chemical contaminants in food. J Microbiol Biotechnol 26:1505–1516
Nie G, Li G, Wang L, Zhang X (2016) Nanocomposites of polymer brush and inorganic nanoparticles: preparation, characterization and application. Polym Chem 7:753–769
Abalde-Cela S, Carregal-Romero S, Paulo Coelho J, Guerrero-Martínez A (2016) Recent progress on colloidal metal nanoparticles as signal enhancers in nanosensing. Adv Colloid Interf Sci 233:255–270
Terenteva EA, Apyari VV, Kochuk EV, Dmitrienko SG, Zolotov YA (2017) Use of silver nanoparticles in spectrophotometry. J Anal Chem 72:1138–1154
Choi I (2016) Recent advances in nanoplasmonic sensors for environmental detection and monitoring. J Nanosci Nanotechnol 16:4274–4283
Yue G, Su S, Li N, Shuai M, Lai X, Astruc D, Zhao P (2016) Gold nanoparticles as sensors in the colorimetric and fluorescence detection of chemical warfare agents. Coord Chem Rev 311:75–84
Apyari VV, Arkhipova VV, Dmitrienko SG, Zolotov YA (2014) Using gold nanoparticles in spectrophotometry. J Anal Chem 69:1–11
Sharma R, Ragavan KV, Thakur MS, Raghavarao KSMS (2015) Recent advances in nanoparticle based aptasensors for food contaminants. Biosens Bioelectron 74:612–627
He Y, Yu H (2015) A novel triangular silver nanoprisms-based surface plasmon resonance assay for free chlorine. Analyst 140:902–906
Zhang L, Li L (2016) Colorimetric detection of hydrogen peroxide using silver nanoparticles with three different morphologies. Anal Methods 8:6691–6695
Millstone JE, Hurst SJ, Metraux GS, Cutler JI, Mirkin CA (2009) Colloidal gold and silver triangular nanoprisms. Small 5:646–664
Zhang LL, Ma FF, Kuang YF, Cheng S, Long YF, Xiao QG (2016) Highly sensitive detection of bovine serum albumin based on the aggregation of triangular silver nanoplates. Spectrochim Acta A 154:98–102
Chen Z, Zhang C, Wu Q, Li K, Tan L (2015) Application of triangular silver nanoplates for colorimetric detection of H2O2. Sensors Actuators B Chem 220:314–317
Peng J, Yang X-H, Ling J, Liu C-J, Zhang X-Q, Cao Q-E, Ding Z-T (2014) Selective detection of mercury (II) by etching the corners of silver triangular nanoplates. Spectrosc Lett 47:549–553
Abkhalimov EV, Timofeev AA, Ershov BG (2018) Electrochemical mechanism of silver nanoprisms transformation in aqueous solutions containing the halide ions. J Nanopart Res 20:26. https://doi.org/10.1007/s11051-018-4133-6
An J, Tang B, Zheng X, Zhou J, Dong F, Xu S, Wang Y, Zhao B, Xu W (2008) Sculpturing effect of chloride ions in shape transformation from triangular to discal silver nanoplates. J Phys Chem C 112:15176–15182
Yang XH, Ling J, Peng J, Cao QE, Ding ZT, Bian LC (2013) A colorimetric method for highly sensitive and accurate detection of iodide by finding the critical color in a color change process using silver triangular nanoplates. Anal Chim Acta 798:74–81
Hsu MS, Cao YW, Wang HW, Pan YS, Lee BH, Huang CL (2010) Time-dependent surface plasmon resonance spectroscopy of silver nanoprisms in the presence of halide ions. Chem Phys Chem 11:1742–1748
Apyari VV, Gorbunova MO, Shevchenko AV, Furletov AA, Volkov PA, Garshev AV, Dmitrienko SG, Zolotov YA (2018) Towards highly selective detection using metal nanoparticles: a case of silver triangular nanoplates and chlorine. Talanta 176:406–411
Gorbunova MO, Shevchenko AV, Apyari VV, Furletov AA, Volkov PA, Garshev AV, Dmitrienko SG (2018) Selective determination of chloride ions using silver triangular nanoplates and dynamic gas extraction. Sensors Actuators B Chem 256:699–705
Katsoulidis AP, He J, Kanatzidis MG (2012) Functional monolithic polymeric organic framework aerogel as reducing and hosting media for ag nanoparticles and application in capturing of iodine vapors. Chem Mater 24:1937–1943
Gorbunova MO, Bayan EM (2017) A rapid field test method for the determination of hydrogen sulfide and sulfides in waters with gas preextraction. J Anal Chem 72:1263–1269
Gorbunova MO, Bayan EM, Shevchenko AV, Kulyaginova MS (2017) Digital colorimetric determination of chlorides in water using gas extraction and methyl orange. Analitika i kontrol’ [Analytics and Control] 21:274–280
Metraux GS, Mirkin CA (2005) Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv Mater 17:412–415
Furletov AA, Apyari VV, Garshev AV, Dmitrienko SG, Zolotov YA (2017) Triangular silver nanoplates as a spectrophotometric reagent for the determination of mercury(II). J Anal Chem 72:1203–1207
Apyari VV, Furletov AA, Garshev AV, Volkov PA, Gorbunova MO, Shevchenko AV (2017) Preparation of reagent indicator papers with silver triangular nanoplates for chemical analysis. Mosc Univ Chem Bull 72:167–173
National pharmacopeia of Russian Federation, Metamizol sodium, FS21000315
National pharmacopeia of Russian Federation, Methods for quantification of vitamins, OFS123001715
Belikov VG (2008) Pharmaceutical chemistry. MEDpress-inform, Moscow
National standard of Russian Federation, Coffee products. Procedure of caffeine mass part measurement, GOST R 51182–98
Costa GO, Feiteira FN, de M. Schuenck H, Pacheco WF (2018) Iodine determination in table salts by digital images analysis. Anal Methods 10:4463–4470
Devouge-Boyer C, Mouda S, Gueguen O, Marcotte S (2018) Determination of iodine in polyamide by inductively-coupled plasma/mass spectrometry. Talanta 189:568–572
Mequanint T, Moges G, Tessma M, Mehretu S (2012) All-solid-state iodide selective electrode for iodimetry of iodized salts and vitamin C. Orient J Chem 28:1547–1555
Gavrilenko NA, Fedan DA, Saranchina NV, Gavrilenko MA (2019) Solid phase colorimetric determination of iodine in food grade salt using polymethacrylate matrix. Food Chem 280:15–19
Novo DLR, Mello JE, Rondan FS, Henn AS, Mello PA, Mesko MF (2019) Bromine and iodine determination in human saliva: challenges in the development of an accurate method. Talanta 191:415–421
Hendawy HAM, Ibrahim AM, Hassan WS, Shalaby A, El-sayed HM (2019) Voltammetric method for simultaneous determination of ascorbic acid, paracetamol and guaifenesin using a sequential experimentation strategy. Microchem J 145:428–434
Salkić M, Selimović A, Keran H (2011) Spectrophotometric determination of l-ascorbic acid in pharmaceutical preparations using glycine as a stabilizer. Eur J Sci Res 53:193–198
Zhao Y, Zhou J, Jia Z, Huo D, Liu Q, Zhong D, Hu Y, Yang M, Bian M, Hou C (2019) In-situ growth of gold nanoparticles on a 3D-network consisting of a MoS2/rGO nanocomposite for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim Acta 186:92. https://doi.org/10.1007/s00604-018-3222-7
Salkić M, Selimović A (2015) Spectrophotometric determination of L-ascorbic acid in pharmaceuticals based on its oxidation by potassium peroxymonosulfate and hydrogen peroxide. Croat Chem Acta 88:73–79
Yu J, Yang W, Xing S, Wang J, Han H, Zhang P, Xiang C, Zhang B (2019) Blended gold/MnO2@BSA nanoparticles for fluorometric and magnetic resonance determination of ascorbic acid. Microchim Acta 186:89. https://doi.org/10.1007/s00604-018-3205-8
Acknowledgements
This work was supported by the Russian Foundation for Basic Research [grant number 18-53-00014-Bel_a] and the Belarusian Republican Foundation for Fundamental Research [grant number F18R-237].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 52 kb)
Rights and permissions
About this article
Cite this article
Gorbunova, M.O., Baulina, A.A., Kulyaginova, M.S. et al. Dynamic gas extraction of iodine in combination with a silver triangular nanoplate-modified paper strip for colorimetric determination of iodine and of iodine-interacting compounds. Microchim Acta 186, 188 (2019). https://doi.org/10.1007/s00604-019-3300-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3300-5