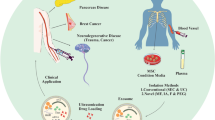
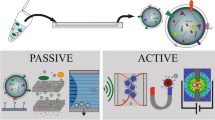
Abstract
Extracellular vesicles are spherical nanoparticles inherently released by almost all cell types. They acquire the cell’s membrane and cytoplasmic characteristics offering abundant identical units that can be captured to recognize the cell of origin. The abundance of vital cell information and multifunctional roles in cellular processes has rendered them attention, particularly as promising biomarkers for disease diagnosis and use in potential drug delivery systems. This review provides insights into standard approaches towards cultivation and isolation of mammalian and bacterial extracellular vesicles. We assess gaps in conventional separation and detection technologies while also tracking developments in ongoing research. The review focuses on highlighting alternative state-of-the-art microfluidic devices that offer avenues for fast, cost-effective, precision-oriented capture and sensing of extracellular vesicles. Combining different detection technologies on an integrated “lab-on-a-chip” system has the prospective to provide customizable opportunities for clinical use of extracellular vesicles in disease diagnostics and therapeutic applications.
Graphical abstract







Similar content being viewed by others
Abbreviations
- AML:
-
acute myeloid leukaemia
- ATPS:
-
aqueous two-phase system
- AuNC:
-
gold nanocluster
- AuR:
-
gold nanorod
- BAW:
-
bulk acoustic waves
- CTC:
-
circulating tumour cell
- DEX:
-
dextran
- DICE:
-
differentiated immunostaining-based characterization of extracellular vesicles
- ELAA:
-
enzyme-linked aptamer assay
- ELISA:
-
enzyme-linked immunosorbent assay
- E. coli :
-
Escherichia coli
- EGFRvIII:
-
epidermal growth factor receptor variant III
- ELAA:
-
enzyme-linked aptamer assay
- EpCAM:
-
epithelial cell adhesion molecule
- GBM:
-
glioblastoma multiforme
- HGSOC:
-
high-grade serous ovarian cancer
- IDE:
-
interdigitated electrodes
- IDT:
-
interdigitated transducers
- iDEP:
-
insulator-based dielectrophoretic
- ISEV:
-
international society for extracellular vesicles
- LOC:
-
lab-on-a-chip
- LOD:
-
limit of detection
- LPS:
-
lipopolysaccharides
- MCA:
-
magnetic colloid antibody
- MNP:
-
magnetic nanoparticles
- MV:
-
membrane vesicles
- MISEV:
-
minimal information for studies of extracellular vesicles
- MVBs:
-
multivesicular bodies
- OMV:
-
outer membrane vesicles
- pAP:
-
para-aminophenol
- PEG:
-
polyethylene glycol
- PMMA:
-
polymethacrylate
- PSM:
-
polystyrene microspheres
- PDL-1:
-
program death ligand 1
- sEVs:
-
small extracellular vesicles
- SEC:
-
size exclusion chromatography
- SAW:
-
surface acoustic wave
- SERS:
-
surface enhance Raman spectroscopy
References
Witwer KW, Théry C (2019) Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles 8:1648167. https://doi.org/10.1080/20013078.2019.1648167
Sager R, Palade GE (1957) Structure and development of the chloroplast in chlamydomonas. I. The normal green cell. J Biophys Biochem Cytol 3:463–488. https://doi.org/10.1083/jcb.3.3.463
Sotelo JR, Porter KR (1959) An Electron microscope study of the rat ovum. J Biophys Biochem Cytol 5:327–342. https://doi.org/10.1083/jcb.5.2.327
Jensen WA (1965) The composition and ultrastructure of the nucellus in cotton. J Ultrastruct Res 13:112–128. https://doi.org/10.1016/S0022-5320(65)80092-2
Takeo K, Uesaka I, Uehira K, Nishiura M (1973) Fine structure of cryptococcus neoformans grown in vitro as observed by freeze-etching. J Bacteriol 113:1442–1448. https://doi.org/10.1128/JB.113.3.1442-1448.1973
Kırbaş OK, Bozkurt BT, Asutay AB, Mat B, Ozdemir B, Öztürkoğlu D, Ölmez H, İşlek Z, Şahin F, Taşlı PN (2019) Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci Rep 9:19159. https://doi.org/10.1038/s41598-019-55477-0
Aaronson S, Behrens U, Orner R, Haines TH (1971) Ultrastructure of intracellular and extracellular vesicles, membranes, and myelin figures produced by ochromonas danica. J Ultrastruct Res 35:418–430. https://doi.org/10.1016/s0022-5320(71)80003-5
Chargaff E, West R (1946) The biological significance of the thromboplastic protein of blood. J Biol Chem 166:189–197
Wolf P (1967) The nature and significance of platelet products in human plasma. Br J Haematol 13:269–288. https://doi.org/10.1111/j.1365-2141.1967.tb08741.x
Anderson HC (1969) Vesicles associated with calcification in the matrix of epiphyseal cartilage. J Cell Biol 41:59–72. https://doi.org/10.1083/jcb.41.1.59
Anderson HC (1967) Electron microscopic studies of induced cartilage development and calcification. J Cell Biol 35:81–101
Bonucci E (1967) Fine structure of early cartilage calcification. J Ultrastruct Res 20:33–50. https://doi.org/10.1016/s0022-5320(67)80034-0
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339. https://doi.org/10.1083/jcb.97.2.329
Pan B-T, Johnstone RM (1983) Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 33:967–978. https://doi.org/10.1016/0092-8674(83)90040-5
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172. https://doi.org/10.1084/jem.183.3.1161
Prangishvili D, Holz I, Stieger E, Nickell S, Kristjansson JK, Zillig W (2000) Sulfolobicins, specific proteinaceous toxins produced by strains of the extremely thermophilic archaeal genus sulfolobus. J Bacteriol 182:2985–2988. https://doi.org/10.1128/JB.182.10.2985-2988.2000
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. https://doi.org/10.1038/ncb1596
Raposo G, Stahl PD (2019) Extracellular vesicles: a new communication paradigm? Nat Rev Mol Cell Biol 20:509–510. https://doi.org/10.1038/s41580-019-0158-7
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman MLD, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DRF, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FAW, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, de Santana EF Junior, de Wever O, del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TAP, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, el Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DCI, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AGE, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ II, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li ITS, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SLN, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen ENM, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BCH, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IKH, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KMA, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PRM, Silva AM, Skowronek A, Snyder OL II, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BWM, van der Grein SG, van Deun J, van Herwijnen MJC, van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MHM, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. https://doi.org/10.1080/20013078.2018.1535750
Lötvall J, Hill AF, Hochberg F, Buzás EI, di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C (2014) Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 3:26913. https://doi.org/10.3402/jev.v3.26913
Negahdaripour M, Owji H, Eskandari S, Zamani M, Vakili B, Nezafat N (2020) Small extracellular vesicles (sEVs): discovery, functions, applications, detection methods and various engineered forms. Expert Opin Biol Ther 21:1–24. https://doi.org/10.1080/14712598.2021.1825677
Harmati M, Gyukity-Sebestyen E, Dobra G, Janovak L, Dekany I, Saydam O, Hunyadi-Gulyas E, Nagy I, Farkas A, Pankotai T, Ujfaludi Z, Horvath P, Piccinini F, Kovacs M, Biro T, Buzas K (2019) Small extracellular vesicles convey the stress-induced adaptive responses of melanoma cells. Sci Rep 9:15329. https://doi.org/10.1038/s41598-019-51778-6
Melidoni A (2020) Small extracellular vesicles combat senescence. Nat Rev Mol Cell Biol 21:498–499. https://doi.org/10.1038/s41580-020-0271-7
Dorward DW, Garon CF (1990) DNA is packaged within membrane-derived vesicles of gram-negative but not gram-positive bacteria. Appl Environ Microbiol 56:1960–1962
Dashper SG, Hendtlass A, Slakeski N, Jackson C, Cross KJ, Brownfield L, Hamilton R, Barr I, Reynolds EC (2000) Characterization of a novel outer membrane hemin-binding protein of porphyromonas gingivalis. J Bacteriol 182:6456–6462
Dineshkumar K, Aparna V, Wu L, Wan J, Abdelaziz MH, Su Z, Wang S, Xu H (2020) Bacterial bug-out bags: outer membrane vesicles and their proteins and functions. J Microbiol 58:531–542. https://doi.org/10.1007/s12275-020-0026-3
Kumar SR, Kimchi ET, Manjunath Y, Gajagowni S, Stuckel AJ, Kaifi JT (2020) RNA cargos in extracellular vesicles derived from blood serum in pancreas associated conditions. Sci Rep 10:2800. https://doi.org/10.1038/s41598-020-59523-0
Musante L, Bontha SV, La Salvia S et al (2020) Rigorous characterization of urinary extracellular vesicles (uEVs) in the low centrifugation pellet - a neglected source for uEVs. Sci Rep 10:3701. https://doi.org/10.1038/s41598-020-60619-w
Conzelmann C, Groß R, Zou M, Krüger F, Görgens A, Gustafsson MO, el Andaloussi S, Münch J, Müller JA (2020) Salivary extracellular vesicles inhibit Zika virus but not SARS-CoV-2 infection. Journal of Extracellular Vesicles 9:1808281. https://doi.org/10.1080/20013078.2020.1808281
de la Torre GC, Goreham RV, Bech Serra JJ et al (2018) “Exosomics”—a review of biophysics, Biology and Biochemistry of Exosomes With a Focus on Human Breast Milk. Front Genet 9. https://doi.org/10.3389/fgene.2018.00092
Severino V, Dumonceau J-M, Delhaye M, et al (2017) Extracellular vesicles in bile as markers of malignant biliary Stenoses. Gastroenterology 153:495-504.e8. https://doi.org/10.1053/j.gastro.2017.04.043
Njock M-S, Guiot J, Henket MA, Nivelles O, Thiry M, Dequiedt F, Corhay JL, Louis RE, Struman I (2019) Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax 74:309–312. https://doi.org/10.1136/thoraxjnl-2018-211897
Liam C-K, Liam Y-S, Wong C-K (2020) Extracellular vesicle-based EGFR genotyping in bronchoalveolar lavage fluid. Transl Lung Cancer Res 9:168–171. https://doi.org/10.21037/tlcr.2020.03.06
Wang R, Gornalusse GG, Kim Y, Pandey U, Hladik F, Vojtech L (2020) Potent restriction of sexual Zika virus infection by the lipid fraction of extracellular vesicles in semen. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.574054
Takeuchi T, Mori K, Sunayama H, Takano E, Kitayama Y, Shimizu T, Hirose Y, Inubushi S, Sasaki R, Tanino H (2020) Antibody-conjugated signaling nanocavities fabricated by dynamic molding for detecting cancers using small extracellular vesicle markers from tears. J Am Chem Soc 142:6617–6624. https://doi.org/10.1021/jacs.9b13874
Dobhal G, Datta A, Ayupova D, Teesdale-Spittle P, Goreham RV (2020) Isolation, characterisation and detection of breath-derived extracellular vesicles. Sci Rep 10:17381. https://doi.org/10.1038/s41598-020-73243-5
Carnino JM, Lee H, Jin Y (2019) Isolation and characterization of extracellular vesicles from broncho-alveolar lavage fluid: a review and comparison of different methods. Respir Res 20:240. https://doi.org/10.1186/s12931-019-1210-z
Maeki M, Kimura N, Sato Y, Harashima H, Tokeshi M (2018) Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv Drug Deliv Rev 128:84–100. https://doi.org/10.1016/j.addr.2018.03.008
Zhu L, Xu N, Zhang Z-L, Zhang T-C (2019) Cell derived extracellular vesicles: from isolation to functionalization and biomedical applications. Biomater Sci 7:3552–3565. https://doi.org/10.1039/C9BM00580C
Hartjes TA, Mytnyk S, Jenster GW, van Steijn V, van Royen M (2019) Extracellular vesicle quantification and characterization: common methods and emerging approaches. Bioengineering 6:7. https://doi.org/10.3390/bioengineering6010007
Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, Garnis C, Daugaard M, Guns E, Hoorfar M, Li ITS (2019) Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng 3:011503. https://doi.org/10.1063/1.5087122
Patel GK, Khan MA, Zubair H, Srivastava SK, Khushman M’, Singh S, Singh AP (2019) Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci Rep 9:5335. https://doi.org/10.1038/s41598-019-41800-2
Sharma S, LeClaire M, Wohlschlegel J, Gimzewski J (2020) Impact of isolation methods on the biophysical heterogeneity of single extracellular vesicles. Sci Rep 10:13327. https://doi.org/10.1038/s41598-020-70245-1
Goreham RV, Ayed Z, Ayupova D, Dobhal G (2019) Extracellular vesicles: nature’s own nanoparticles. In: Comprehensive Nanoscience and Nanotechnology. Elsevier, pp. 27–48
Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CMJ, Zhang L (2015) Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett 15:1403–1409. https://doi.org/10.1021/nl504798g
Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, Wang Y (2020) Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release 323:253–268. https://doi.org/10.1016/j.jconrel.2020.04.031
Kim OY, Park HT, Dinh NTH, Choi SJ, Lee J, Kim JH, Lee SW, Gho YS (2017) Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun 8:626. https://doi.org/10.1038/s41467-017-00729-8
Van Deun J, Mestdagh P, Sormunen R et al (2014) The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 3:24858. https://doi.org/10.3402/jev.v3.24858
Yang F, Liao X, Tian Y, Li G (2017) Exosome separation using microfluidic systems: size-based, immunoaffinity-based and dynamic methodologies. Biotechnol J 12:1600699. https://doi.org/10.1002/biot.201600699
Rubin D, Christy C (2002) Selecting the right ultrafiltration membrane for biopharmaceutical applications. Pharm Technol Eur 14:39
Monguió-Tortajada M, Gálvez-Montón C, Bayes-Genis A, Roura S, Borràs FE (2019) Extracellular vesicle isolation methods: rising impact of size-exclusion chromatography. Cell Mol Life Sci 76:2369–2382. https://doi.org/10.1007/s00018-019-03071-y
Brownlee Z, Lynn KD, Thorpe PE, Schroit AJ (2014) A novel “salting-out” procedure for the isolation of tumor-derived exosomes. J Immunol Methods 407:120–126. https://doi.org/10.1016/j.jim.2014.04.003
Klimentová J, Stulík J (2015) Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiol Res 170:1–9. https://doi.org/10.1016/j.micres.2014.09.006
Santucci L, Bruschi M, Del Zotto G et al (2019) Biological surface properties in extracellular vesicles and their effect on cargo proteins. Sci Rep 9:13048. https://doi.org/10.1038/s41598-019-47598-3
Chen S, Shiesh S-C, Lee G-B, Chen C (2020) Two-step magnetic bead-based (2MBB) techniques for immunocapture of extracellular vesicles and quantification of microRNAs for cardiovascular diseases: a pilot study. PLoS One 15:e0229610. https://doi.org/10.1371/journal.pone.0229610
Wan Y, Cheng G, Liu X, Hao SJ, Nisic M, Zhu CD, Xia YQ, Li WQ, Wang ZG, Zhang WL, Rice SJ, Sebastian A, Albert I, Belani CP, Zheng SY (2017) Rapid magnetic isolation of extracellular vesicles via lipid-based nanoprobes. Nat Biomed Eng 1:1–11. https://doi.org/10.1038/s41551-017-0058
Zhang K, Yue Y, Wu S, Liu W, Shi J, Zhang Z (2019) Rapid capture and nondestructive release of extracellular vesicles using aptamer-based magnetic isolation. ACS Sens 4:1245–1251. https://doi.org/10.1021/acssensors.9b00060
Ishida T, Hashimoto T, Masaki K, Funabashi H, Hirota R, Ikeda T, Tajima H, Kuroda A (2020) Application of peptides with an affinity for phospholipid membranes during the automated purification of extracellular vesicles. Sci Rep 10:1–12. https://doi.org/10.1038/s41598-020-75561-0
Chen Y-S, Ma Y-D, Chen C, Shiesh SC, Lee GB (2019) An integrated microfluidic system for on-chip enrichment and quantification of circulating extracellular vesicles from whole blood. Lab Chip 19:3305–3315. https://doi.org/10.1039/C9LC00624A
Yang J, Pan B, Zeng F, He B, Gao Y, Liu X, Song Y (2021) Magnetic colloid antibodies accelerate small extracellular vesicles isolation for point-of-care diagnostics. Nano Lett 21:2001–2009. https://doi.org/10.1021/acs.nanolett.0c04476
Alves NJ, Turner KB, DiVito KA et al (2017) Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a his-tag mutant. Res Microbiol 168:139–146. https://doi.org/10.1016/j.resmic.2016.10.001
Xue F, Chen Y, Wen Y, Abhange K, Zhang W, Cheng G, Quinn Z, Mao W, Wan Y (2021) Isolation of extracellular vesicles with multivalent aptamers. Analyst 146:253–261. https://doi.org/10.1039/D0AN01420F
Liangsupree T, Multia E, Riekkola M-L (1636) Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A 2021:461773. https://doi.org/10.1016/j.chroma.2020.461773
Royo F, Théry C, Falcón-Pérez JM, Nieuwland R, Witwer KW (2020) Methods for separation and characterization of extracellular vesicles: results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells 9:1955. https://doi.org/10.3390/cells9091955
Salmond N, Williams CK (2021) Isolation and characterization of extracellular vesicles for clinical applications in cancer – time for standardization? Nanoscale Adv. https://doi.org/10.1039/D0NA00676A
Szatanek R, Baran J, Siedlar M, Baj-Krzyworzeka M (2015) Isolation of extracellular vesicles: determining the correct approach (review). Int J Mol Med 36:11–17. https://doi.org/10.3892/ijmm.2015.2194
Doyle LM, Wang MZ (2019) Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells 8:727. https://doi.org/10.3390/cells8070727
Cufaro MC, Pieragostino D, Lanuti P, et al (2019) Extracellular vesicles and their potential use in monitoring cancer progression and therapy: the contribution of proteomics. J Oncol. https://www.hindawi.com/journals/jo/2019/1639854/. Accessed 3 Mar 2021
Gandham S, Su X, Wood J, Nocera AL, Alli SC, Milane L, Zimmerman A, Amiji M, Ivanov AR (2020) Technologies and standardization in research on extracellular vesicles. Trends Biotechnol 38:1066–1098. https://doi.org/10.1016/j.tibtech.2020.05.012
Sunkara V, Woo H-K, Cho Y-K (2016) Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst 141:371–381. https://doi.org/10.1039/C5AN01775K
Serrano-Pertierra E, Oliveira-Rodríguez M, Rivas M, Oliva P, Villafani J, Navarro A, Blanco-López M, Cernuda-Morollón E (2019) Characterization of plasma-derived extracellular vesicles isolated by different methods: a comparison study. Bioengineering 6:8. https://doi.org/10.3390/bioengineering6010008
Tiwari S, Kumar V, Randhawa S, Verma SK (2021) Preparation and characterization of extracellular vesicles. Am J Reprod Immunol 85:e13367. https://doi.org/10.1111/aji.13367
Serrano-Pertierra E, Oliveira-Rodríguez M, Matos M, Gutiérrez G, Moyano A, Salvador M, Rivas M, Blanco-López MC (2020) Extracellular vesicles: current analytical techniques for detection and quantification. Biomolecules 10:824. https://doi.org/10.3390/biom10060824
Tran PH-L, Xiang D, Nguyen TN-G, Tran TTD, Chen Q, Yin W, Zhang Y, Kong L, Duan A, Chen K, Sun M, Li Y, Hou Y, Zhu Y, Ma Y, Jiang G, Duan W (2020) Aptamer-guided extracellular vesicle theranostics in oncology. Theranostics 10:3849–3866. https://doi.org/10.7150/thno.39706
Chen Y, Wang Z, Liu Y, Wang X, Li Y, Ma P, Gu B, Li H (2018) Recent advances in rapid pathogen detection method based on biosensors. Eur J Clin Microbiol Infect Dis 37:–1037. https://doi.org/10.1007/s10096-018-3230-x
Rajapaksha P, Elbourne A, Gangadoo S, Brown R, Cozzolino D, Chapman J (2019) A review of methods for the detection of pathogenic microorganisms. Analyst 144:396–411. https://doi.org/10.1039/C8AN01488D
Ali AA, Altemimi AB, Alhelfi N, Ibrahim SA (2020) Application of biosensors for detection of pathogenic food Bacteria: a review. Biosensors 10:58. https://doi.org/10.3390/bios10060058
Riu J, Giussani B (2020) Electrochemical biosensors for the detection of pathogenic bacteria in food. TrAC Trends Anal Chem 126:115863. https://doi.org/10.1016/j.trac.2020.115863
Lu J, Pang J, Chen Y, Dong Q, Sheng J, Luo Y, Lu Y, Lin B, Liu T (2019) Application of microfluidic chips in separation and analysis of extracellular vesicles in liquid biopsy for cancer. Micromachines 10:390. https://doi.org/10.3390/mi10060390
Srivastava A, Amreddy N, Razaq M, et al (2018) Exosomes as theranostics for lung cancer. In: Advances in Cancer Research. Elsevier, pp. 1–33
Tian F, Liu C, Lin L, Chen Q, Sun J (2019) Microfluidic analysis of circulating tumor cells and tumor-derived extracellular vesicles. TrAC Trends Anal Chem 117:128–145. https://doi.org/10.1016/j.trac.2019.05.013
Deng Z, Wang Y, Hu L, Zhu Q, Chen Y, Chen JJ, Chen J, Zhang T, Seo TS, Liu F (2020) A facile, rapid, high-throughput extracellular vesicles analytical platform for cancer detection. Anal Chim Acta 1138:132–140. https://doi.org/10.1016/j.aca.2020.08.053
Dobhal G, Ayupova D, Laufersky G, Ayed Z, Nann T, Goreham R (2018) Cadmium-free quantum dots as fluorescent labels for exosomes. Sensors 18:3308. https://doi.org/10.3390/s18103308
Ichzan AM, Lee S, San Fang C, Nandhakumar P, Ha H, Joo JM, Kim KS, Yang H (2019) Use of a phosphatase-like DT-diaphorase label for the detection of outer membrane vesicles. Anal Chem 91:4680–4686. https://doi.org/10.1021/acs.analchem.9b00064
Yu X, He L, Pentok M, Yang H, Yang Y, Li Z, He N, Deng Y, Li S, Liu T, Chen X, Luo H (2019) An aptamer-based new method for competitive fluorescence detection of exosomes. Nanoscale 11:15589–15595. https://doi.org/10.1039/C9NR04050A
Smith MK, Martin-Peralta DG, Pivak PA, Mirica KA (2017) Fabrication of solid-state gas sensors by drawing: an undergraduate and high school introduction to functional nanomaterials and chemical detection. J Chem Educ 94:1933–1938. https://doi.org/10.1021/acs.jchemed.6b00997
Cui F, Zhou Z, Zhou HS (2020) Review—measurement and analysis of cancer biomarkers based on electrochemical biosensors. J Electrochem Soc 167:037525. https://doi.org/10.1149/2.0252003JES
Acero Sánchez JLl, Joda H, Henry OYF, et al (2017) Electrochemical genetic profiling of single cancer cells. Anal Chem 89:3378–3385. https://doi.org/10.1021/acs.analchem.6b03973
Zheng D, Zhu X, Ding X, Zhu X, Yin Y, Li G (2013) Sensitive detection of CD147/EMMPRIN and its expression on cancer cells with electrochemical technique. Talanta 105:187–191. https://doi.org/10.1016/j.talanta.2012.11.060
Zhou J, Cheng K, Chen X, Yang R, Lu M, Ming L, Chen Y, Lin Z, Chen D (2020) Determination of soluble CD44 in serum by using a label-free aptamer based electrochemical impedance biosensor. Analyst 145:460–465. https://doi.org/10.1039/C9AN01764J
Hansen JA, Wang J, Kawde A-N, Xiang Y, Gothelf KV, Collins G (2006) Quantum-dot/aptamer-based ultrasensitive multi-analyte electrochemical biosensor. J Am Chem Soc 128:2228–2229. https://doi.org/10.1021/ja060005h
Kumar J, Dsouza S (2007) Preparation of PVA membrane for immobilization of GOD for glucose biosensor. Talanta S003991400700745X. https://doi.org/10.1016/j.talanta.2007.10.048
Wang J, Musameh M, Lin Y (2003) Solubilization of carbon nanotubes by Nafion toward the preparation of amperometric biosensors. J Am Chem Soc 125:2408–2409. https://doi.org/10.1021/ja028951v
Balaji A, Zhang J (2017) Electrochemical and optical biosensors for early-stage cancer diagnosis by using graphene and graphene oxide. Cancer Nanotechnol 8:10. https://doi.org/10.1186/s12645-017-0035-z
Wang L, Xiong Q, Xiao F, Duan H (2017) 2D nanomaterials based electrochemical biosensors for cancer diagnosis. Biosens Bioelectron 89:136–151. https://doi.org/10.1016/j.bios.2016.06.011
Li J, Zhang J, Chen Y, Kawazoe N, Chen G (2017) TEMPO-conjugated gold nanoparticles for reactive oxygen species scavenging and regulation of stem cell differentiation. ACS Appl Mater Interfaces 9:35683–35692. https://doi.org/10.1021/acsami.7b12486
Saxena U, Das AB (2016) Nanomaterials towards fabrication of cholesterol biosensors: key roles and design approaches. Biosens Bioelectron 75:196–205. https://doi.org/10.1016/j.bios.2015.08.042
Koo KM, Carrascosa LG, Shiddiky MJA, Trau M (2016) Poly(a) extensions of miRNAs for amplification-free electrochemical detection on screen-printed gold electrodes. Anal Chem 88:2000–2005. https://doi.org/10.1021/acs.analchem.5b04795
Su X-L, Li Y (2004) A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of escherichia coli O157:H7. Biosens Bioelectron 19:563–574. https://doi.org/10.1016/S0956-5663(03)00254-9
Zhuo Y, Yuan R, Chai Y, Tang D, Zhang Y, Wang N, Li X, Zhu Q (2005) A reagentless amperometric immunosensor based on gold nanoparticles/thionine/Nafion-membrane-modified gold electrode for determination of α-1-fetoprotein. Electrochem Commun 7:355–360. https://doi.org/10.1016/j.elecom.2005.02.001
Xu X, Makaraviciute A, Kumar S, Wen C, Sjödin M, Abdurakhmanov E, Danielson UH, Nyholm L, Zhang Z (2019) Structural changes of mercaptohexanol self-assembled monolayers on gold and their influence on impedimetric aptamer sensors. Anal Chem 91:14697–14704. https://doi.org/10.1021/acs.analchem.9b03946
Vargis VS, Chandhana JP, Suneesh PV, Nair B, Satheesh Babu TG (2019) Voltammetric immunosensing platform based on dual signal amplification using gold nanoparticle labels. IOP Conf Ser: Mater Sci Eng 577:012103. https://doi.org/10.1088/1757-899X/577/1/012103
Braiek M, Rokbani K, Chrouda A, Mrabet B, Bakhrouf A, Maaref A, Jaffrezic-Renault N (2012) An electrochemical immunosensor for detection of Staphylococcus aureus bacteria based on immobilization of antibodies on self-assembled monolayers-functionalized gold electrode. Biosensors 2:417–426. https://doi.org/10.3390/bios2040417
Bakhtiari H, Palizban A, Khanahmad H, Mofid M (2020) Aptamer-based approaches for in vitro molecular detection of cancer. Res Pharma Sci 15:107–122. https://doi.org/10.4103/1735-5362.283811
Balamurugan S, Obubuafo A, Soper SA, Spivak DA (2008) Surface immobilization methods for aptamer diagnostic applications. Anal Bioanal Chem 390:1009–1021. https://doi.org/10.1007/s00216-007-1587-2
Villalonga A, Pérez-Calabuig AM, Villalonga R (2020) Electrochemical biosensors based on nucleic acid aptamers. Anal Bioanal Chem 412:55–72. https://doi.org/10.1007/s00216-019-02226-x
Zhang H, Zhou Y, Luo D, Liu J, Yang E, Yang G, Feng G, Chen Q, Wu L (2021) Immunoassay-aptasensor for the determination of tumor-derived exosomes based on the combination of magnetic nanoparticles and hybridization chain reaction. RSC Adv 11:4983–4990. https://doi.org/10.1039/D0RA10159A
Yadav S, Boriachek K, Islam MN, Lobb R, Möller A, Hill MM, Hossain MSA, Nguyen NT, Shiddiky MJA (2017) An electrochemical method for the detection of disease-specific Exosomes. ChemElectroChem 4:967–971. https://doi.org/10.1002/celc.201600391
Shin H-S, Gedi V, Kim J-K, Lee D (2019) Detection of gram-negative bacterial outer membrane vesicles using DNA aptamers. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-49755-0
Webber J, Clayton A (2013) How pure are your vesicles? J Extracell Vesicles 2. https://doi.org/10.3402/jev.v2i0.19861
Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP (2018) Isolation of extracellular vesicles: general methodologies and latest trends. In: BioMed Research International. https://www.hindawi.com/journals/bmri/2018/8545347/. Accessed 13 Nov 2020
Swieszkowski W, Dokmeci MR, Khademhosseini A (2020) Microfluidics in biofabrication. Biofabrication 12:030201. https://doi.org/10.1088/1758-5090/ab7e75
Whitesides GM (2006) The origins and the future of microfluidics. Nature 442:368–373. https://doi.org/10.1038/nature05058
Guo S-C, Tao S-C, Dawn H (2018) Microfluidics-based on-a-chip systems for isolating and analysing extracellular vesicles. J Extracell Vesicles 7. https://doi.org/10.1080/20013078.2018.1508271
Liga A, Vliegenthart ADB, Oosthuyzen W, Dear JW, Kersaudy-Kerhoas M (2015) Exosome isolation: a microfluidic road-map. Lab Chip 15:2388–2394. https://doi.org/10.1039/C5LC00240K
Gholizadeh S, Shehata Draz M, Zarghooni M, Sanati-Nezhad A, Ghavami S, Shafiee H, Akbari M (2017) Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: current status and future directions. Biosens Bioelectron 91:588–605. https://doi.org/10.1016/j.bios.2016.12.062
Vaidyanathan R, Naghibosadat M, Rauf S, Korbie D, Carrascosa LG, Shiddiky MJA, Trau M (2014) Detecting Exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem 86:11125–11132. https://doi.org/10.1021/ac502082b
Zhao Z, Yang Y, Zeng Y, He M (2016) A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16:489–496. https://doi.org/10.1039/C5LC01117E
Lewis JM, Vyas AD, Qiu Y, Messer KS, White R, Heller MJ (2018) Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano 12:3311–3320. https://doi.org/10.1021/acsnano.7b08199
Liang L-G, Kong M-Q, Zhou S, Sheng YF, Wang P, Yu T, Inci F, Kuo WP, Li LJ, Demirci U, Wang SQ (2017) An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci Rep 7:46224. https://doi.org/10.1038/srep46224
Woo H-K, Sunkara V, Park J, Kim TH, Han JR, Kim CJ, Choi HI, Kim YK, Cho YK (2017) Exodisc for rapid, size-selective, and efficient isolation and analysis of Nanoscale extracellular vesicles from biological samples. ACS Nano 11:1360–1370. https://doi.org/10.1021/acsnano.6b06131
Idaszek J, Costantini M, Karlsen TA, Jaroszewicz J, Colosi C, Testa S, Fornetti E, Bernardini S, Seta M, Kasarełło K, Wrzesień R, Cannata S, Barbetta A, Gargioli C, Brinchman JE, Święszkowski W (2019) 3D bioprinting of hydrogel constructs with cell and material gradients for the regeneration of full-thickness chondral defect using a microfluidic printing head. Biofabrication 11:044101. https://doi.org/10.1088/1758-5090/ab2622
Li Y, Zhang T, Pang Y, Li L, Chen ZN, Sun W (2019) 3D bioprinting of hepatoma cells and application with microfluidics for pharmacodynamic test of Metuzumab. Biofabrication 11:034102. https://doi.org/10.1088/1758-5090/ab256c
Lee K, Shao H, Weissleder R, Lee H (2015) Acoustic purification of extracellular microvesicles. ACS Nano 9:2321–2327. https://doi.org/10.1021/nn506538f
Evander M, Gidlöf O, Olde B, Erlinge D, Laurell T (2015) Non-contact acoustic capture of microparticles from small plasma volumes. Lab Chip 15:2588–2596. https://doi.org/10.1039/C5LC00290G
Shi L, Esfandiari L (2021) An electrokinetically-driven microchip for rapid entrapment and detection of nanovesicles. Micromachines 12:11. https://doi.org/10.3390/mi12010011
Casadei L, Choudhury A, Sarchet P, Mohana Sundaram P, Lopez G, Braggio D, Balakirsky G, Pollock R, Prakash S (2021) Cross-flow microfiltration for isolation, selective capture and release of liposarcoma extracellular vesicles. J Extracell Vesicles 10:e12062. https://doi.org/10.1002/jev2.12062
Sunkara V, Kim C-J, Park J, Woo HK, Kim D, Ha HK, Kim MH, Son Y, Kim JR, Cho YK (2019) Fully automated, label-free isolation of extracellular vesicles from whole blood for cancer diagnosis and monitoring. Theranostics 9:1851–1863. https://doi.org/10.7150/thno.32438
Tayebi M, Zhou Y, Tripathi P, Chandramohanadas R, Ai Y (2020) Exosome purification and analysis using a facile microfluidic hydrodynamic trapping device. Anal Chem 92:10733–10742. https://doi.org/10.1021/acs.analchem.0c02006
Zhou S, Hu T, Zhang F, Tang D, Li D, Cao J, Wei W, Wu Y, Liu S (2020) Integrated microfluidic device for accurate extracellular vesicle quantification and protein markers analysis directly from human whole blood. Anal Chem 92:1574–1581. https://doi.org/10.1021/acs.analchem.9b04852
Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, Wei JY, Hu G, Nie G, Sun J (2017) Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 11:6968–6976. https://doi.org/10.1021/acsnano.7b02277
Zhang Y, Tong X, Yang L, Yin R, Li Y, Zeng D, Wang X, Deng K (2021) A herringbone mixer based microfluidic device HBEXO-chip for purifying tumor-derived exosomes and establishing miRNA signature in pancreatic cancer. Sensors Actuators B Chem 332:129511. https://doi.org/10.1016/j.snb.2021.129511
Reátegui E, van der Vos KE, Lai CP, Zeinali M, Atai NA, Aldikacti B, Floyd FP Jr, H. Khankhel A, Thapar V, Hochberg FH, Sequist LV, Nahed BV, S. Carter B, Toner M, Balaj L, T. Ting D, Breakefield XO, Stott SL (2018) Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat Commun 9:175. https://doi.org/10.1038/s41467-017-02261-1
Mathew DG, Beekman P, Lemay SG, Zuilhof H, le Gac S, van der Wiel WG (2020) Electrochemical detection of tumor-derived extracellular vesicles on nanointerdigitated electrodes. Nano Lett 20:820–828. https://doi.org/10.1021/acs.nanolett.9b02741
Beekman P, Enciso-Martinez A, Rho HS, Pujari SP, Lenferink A, Zuilhof H, Terstappen LWMM, Otto C, le Gac S (2019) Immuno-capture of extracellular vesicles for individual multi-modal characterization using AFM, SEM and Raman spectroscopy. Lab Chip 19:2526–2536. https://doi.org/10.1039/C9LC00081J
Friend J, Yeo LY (2011) Microscale acoustofluidics: microfluidics driven via acoustics and ultrasonics. Rev Mod Phys 83:647–704. https://doi.org/10.1103/RevModPhys.83.647
Dual J, Schwarz T (2012) Acoustofluidics 3: continuum mechanics for ultrasonic particle manipulation. Lab Chip 12:244–252. https://doi.org/10.1039/C1LC20837C
Yeo LY, Friend JR (2009) Ultrafast microfluidics using surface acoustic waves. Biomicrofluidics 3:012002. https://doi.org/10.1063/1.3056040
Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ (2017) Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A 114:10584–10589. https://doi.org/10.1073/pnas.1709210114
Evander M, Gidlöf O, Olde B, Erlinge D, Laurell T (2015) Non-contact acoustic capture of microparticles from small plasma volumes. Lab Chip 15:2588–2596. https://doi.org/10.1039/C5LC00290G
Li P, Mao Z, Peng Z, Zhou L, Chen Y, Huang PH, Truica CI, Drabick JJ, el-Deiry WS, Dao M, Suresh S, Huang TJ (2015) Acoustic separation of circulating tumor cells. Proc Natl Acad Sci U S A 112:4970–4975. https://doi.org/10.1073/pnas.1504484112
Wu M, Ozcelik A, Rufo J, Wang Z, Fang R, Jun Huang T (2019) Acoustofluidic separation of cells and particles. Microsyst Nanoeng 5:32. https://doi.org/10.1038/s41378-019-0064-3
Strohm EM, Gnyawali V, Sebastian JA, Ngunjiri R, Moore MJ, Tsai SSH, Kolios MC (2019) Sizing biological cells using a microfluidic acoustic flow cytometer. Sci Rep 9:4775. https://doi.org/10.1038/s41598-019-40895-x
Ahmad R, Destgeer G, Afzal M, Park J, Ahmed H, Jung JH, Park K, Yoon TS, Sung HJ (2017) Acoustic wave-driven functionalized particles for aptamer-based target biomolecule separation. Anal Chem 89:13313–13319. https://doi.org/10.1021/acs.analchem.7b03474
Chen W, Li H, Su W, Qin J (2019) Microfluidic device for on-chip isolation and detection of circulating exosomes in blood of breast cancer patients. Biomicrofluidics 13:054113. https://doi.org/10.1063/1.5110973
Lo T-W, Zhu Z, Purcell E, Watza D, Wang J, Kang YT, Jolly S, Nagrath D, Nagrath S (2020) Microfluidic device for high-throughput affinity-based isolation of extracellular vesicles. Lab Chip 20:1762–1770. https://doi.org/10.1039/C9LC01190K
Kamyabi N, Abbasgholizadeh R, Maitra A, Ardekani A, Biswal SL, Grande-Allen KJ (2020) Isolation and mutational assessment of pancreatic cancer extracellular vesicles using a microfluidic platform. Biomed Microdevices 22:23. https://doi.org/10.1007/s10544-020-00483-7
Yang Q, Cheng L, Hu L, Lou D, Zhang T, Li J, Zhu Q, Liu F (2020) An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens Bioelectron 163:112290. https://doi.org/10.1016/j.bios.2020.112290
Dorayappan KDP, Gardner ML, Hisey CL, Zingarelli RA, Smith BQ, Lightfoot MDS, Gogna R, Flannery MM, Hays J, Hansford DJ, Freitas MA, Yu L, Cohn DE, Selvendiran K (2019) A microfluidic chip enables isolation of exosomes and establishment of their protein profiles and associated signaling pathways in ovarian cancer. Cancer Res 79:3503–3513. https://doi.org/10.1158/0008-5472.CAN-18-3538
Kang Y-T, Hadlock T, Jolly S, Nagrath S (2020) Extracellular vesicles on demand (EVOD) chip for screening and quantification of cancer-associated extracellular vesicles. Biosens Bioelectron 168:112535. https://doi.org/10.1016/j.bios.2020.112535
Kang Y-T, Purcell E, Hadlock T, Lo TW, Mutukuri A, Jolly S, Nagrath S (2019) Multiplex isolation and profiling of extracellular vesicles using a microfluidic DICE device. Analyst 144:5785–5793. https://doi.org/10.1039/C9AN01235D
Kang Y-T, Purcell E, Palacios-Rolston C, Lo TW, Ramnath N, Jolly S, Nagrath S (2019) Isolation and profiling of circulating tumor-associated exosomes using extracellular vesicular lipid–protein binding affinity based microfluidic device. Small 15:1903600. https://doi.org/10.1002/smll.201903600
Jalali M, Hosseini II, AbdelFatah T et al (2021) Plasmonic nanobowtiefluidic device for sensitive detection of glioma extracellular vesicles by Raman spectrometry. Lab Chip 21:855–866. https://doi.org/10.1039/D0LC00957A
Han BH, Kim S, Seo G, Heo Y, Chung S, Kang JY (2020) Isolation of extracellular vesicles from small volumes of plasma using a microfluidic aqueous two-phase system. Lab Chip 20:3552–3559. https://doi.org/10.1039/D0LC00345J
Zeng L, Chen X, Du J et al (2021) Label-free separation of nanoscale particles by an ultrahigh gradient magnetic field in a microfluidic device. Nanoscale 13:4029–4037. https://doi.org/10.1039/D0NR08383F
Chen Z, Yang Y, Yamaguchi H, Hung MC, Kameoka J (2020) Isolation of cancer-derived extracellular vesicle subpopulations by a size-selective microfluidic platform. Biomicrofluidics 14:034113. https://doi.org/10.1063/5.0008438
Dehghani M, Lucas K, Flax J, McGrath J, Gaborski T (2019) Tangential flow microfluidics for the capture and release of nanoparticles and extracellular vesicles on conventional and ultrathin membranes. Adv Mater Technol 4:1900539. https://doi.org/10.1002/admt.201900539
Han Z, Peng C, Yi J, Zhang D, Xiang X, Peng X, Su B, Liu B, Shen Y, Qiao L (2021) Highly efficient exosome purification from human plasma by tangential flow filtration based microfluidic chip. Sensors Actuators B Chem 333:129563. https://doi.org/10.1016/j.snb.2021.129563
Liu C, Zhao J, Tian F, Chang J, Zhang W, Sun J (2019) λ-DNA- and aptamer-mediated sorting and analysis of extracellular vesicles. J Am Chem Soc 141:3817–3821. https://doi.org/10.1021/jacs.9b00007
Suwatthanarak T, Adiyasa Thiodorus I, Tanaka M et al (2021) Microfluidic-based capture and release of cancer-derived exosomes via peptide–nanowire hybrid interface. Lab Chip 21:597–607. https://doi.org/10.1039/D0LC00899K
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Malhotra, S., Amin, Z.M., Dobhal, G. et al. Novel devices for isolation and detection of bacterial and mammalian extracellular vesicles. Microchim Acta 188, 139 (2021). https://doi.org/10.1007/s00604-021-04790-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04790-5