Abstract
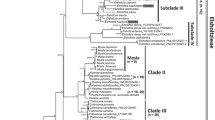
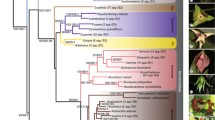
Biebersteiniaceae comprise a single genus with four species of perennial herbs occurring from central Asia to Greece. A previous molecular phylogenetic study placed one of the species in an isolated position in Sapindales, while morphological studies had placed Biebersteinia in or near Geraniaceae, albeit doubtfully. We tested the monophyly and placement of the family with data from the chloroplast genes rbcL and atpB obtained for all four species, other major clades of Sapindales and outgroups for a total of up to 114 taxa. Parsimony, Bayesian, and likelihood analyses place Biebersteinia in Sapindales, possibly as sister to the other eight families. Strict and relaxed molecular clocks constrained with fossils of Biebersteinia and up to eight other Sapindales suggest that the Biebersteinia crown group diversified in the Oligocene and Miocene, while the stem lineage dates back to the Late Paleocene. Ages for other sapindalean families are earlier than those obtained in more sparsely sampled analyses, although estimates for Burseraceae agree surprisingly well. Ancestral area analyses suggest that Biebersteinia expanded from the eastern part of its range (i.e. Tibet and Inner Mongolia) to the west, although analyses are hampered by the unclear sister group relationships.
Similar content being viewed by others
References
(1998). An ordinal classification for the families of flowering plants. Ann. Missouri Bot. Gard. 85: 531–553
(2003). An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 141: 399–436
Bakker F. R., Vassiliades D. D., Morton C. and Savolainen V. (1998). Phylogenetic relationships of Biebersteinia Stephan (Geraniaceae) inferred from rbcL and atpB sequence comparisons. Bot. J. Linn. Soc. 127: 149–158
Barron E. J. and Harrison C. G. A. (1980). An analysis of past plate motions; the South Atlantic and Indian oceans. In: Davies, P. A. and Runcorn, S. K. (eds) Mechanisms of continental drift and plate tectonics, pp 89–109. Academic Press, London
Bell C. D. and Donoghue M. J. (2003). Phylogeny and biogeography of Morinaceae (Dipsacales) based on nuclear and chloroplast DNA sequences. Org. Divers. Evol. 3: 227–237
Boissier E. (1867) Biebersteiniae. In: Flora Orientalis Vol. 1. H. Georg, Basilee, pp. 899–900.
Bremer K. (1992). Ancestral areas: a cladistic reinterpretation of the center of origin concept. Syst. Biol. 41: 436–445
Brenner G. J. (1996). Evidence for the earliest stage of angiosperm pollen evolution: A paleoequatorial section from Israel. In: Taylor, D. W. and Hickey, L. J. (eds) Flowering plant origin, evolution and phylogeny, pp 91–115. Chapman and Hall, New York
Chase M. W., Morton C. M. and Kallunki J. A. (1999). Phylogenetic relationships of Rutaceae: a cladistic analysis of the subfamilies using evidence from rbcL and atpB sequence variation. Amer. J. Bot. 86: 1191–1199
Corbett S. L. and Manchester S. R. (2004). Phytogeography and fossil history of Ailanthus (Simaroubaceae). Int. J. Pl. Sci. 165: 671–690
DeVore M. L., Kenrick P., Pigg K. B., Ketcham R. A. (2005) CT-Scanning the London Clay: an excellent noninvasive technique for studying pyritized fossil fruits. Abstract 122, Botany 2005.
Doyle J. J. and Doyle J. L. (1987). A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochem. Bull. 19: 11–15
Endlicher S. L. (1841). Biebersteiniaceae. In: Enchiridion Botanicum. Leipzig.
Farsam H., Amanlou M., Reza Dehpour A. and Jahaniani F. (2000). Anti-inflammatory and analgesic activity of Biebersteinia multifida DC. root extract. J. Ethnopharmacol. 71: 443–447
Fay M. F., Bayer C., Alverson W., Bruijn A. Y. de, Swensen S. M. and Chase M. W. (1998). Plastid rbcL sequences indicate a close affinity between Diegodendron and Bixa. Taxon 47: 43–50
Felsenstein J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791
Fernando E. S., Gadek P. A. and Quinn C. J. (1995). Simaroubaceae, an artificial construct: evidence from rbcL sequence variation. Amer. J. Bot. 82: 92–103
Fitch W. M. (1971). Toward defining the course of evolution: minimal change for a specific tree topology. Syst. Zool. 20: 406–416
Ghosh P. K. and Roy S. K. (1979). Chisochetonoxylon benegalensis gen. et sp. nov., a new fossil wood of Meliaceae from the Tertiary beds of Birbhum District, West Bengal, India. Curr. Sci. 48: 737–739
Graham A. and Jarzen D. M. (1969). Studies in Neotropical paleobotany. I. The Oligocene communities of Puerto Rico. Ann. Missouri Bot. Gard. 56: 308–357
Gravendeel B., Schuiteman A. and de Vogel E. F. (2005). Molecular dating and vicariance analysis of Coelogyninae (Orchidaceae). In: Bakker, F. T., Chatrou, L. W., Gravendeel, B., and Pelser, P. B. (eds) Plant-level systematics, new perspectives on pattern & process, pp 131–148. Gantner Verlag, Liechtentstein
Greenham J., Vassiliades D. D., Harborne J. B., Williams C. A., Eagles J., Grayer R. J. and Veitch N. C. (2001). A distinctive flavonoid chemistry for the anomalous genus Biebersteinia. Phytochemistry 56: 87–91
Grierson A. J. C. and Long D. G. (1987). Flora of Bhutan, vol 1, part 3. Royal Botanic Garden Edinburgh, Edinburgh
Hoot S. B., Culham A. and Crane P. R. (1995). The utility of atpB gene sequences in resolving phylogenetic relationships: Comparison within rbcL and 18S ribosomal DNA sequences in the Lardizabalaceae. Ann. Missouri Bot. Gard. 82: 194–207
Hughes N. F. (1994). The enigma of angiosperm origins. Cambridge Palaeobiology Series 1. Cambridge University Press, Cambridge
Knuth R. (1912). Biebersteinia Steph. In: Engler, A. (eds) Das Pflanzenreich IV, Vol. 129, pp 546–549. H. R. Engelmann, Berlin
Lee T.-Y. and Lawver L. A. (1995). Cenozoic plate reconstruction of the southeast Asia regions. Tectonophysics 251: 85–138
Linder H. P., Hardy C. R. and Rutschmann F. (2005). Taxon sampling effects in molecular clock dating: An example from the African Restionaceae. Molec. Phylogenet. Evol. 35: 569–582
MacGinitie H. D. (1969). The Eocene Green River flora of northwestern Colorado and northeastern Utah. Univ. Calif. Publ. Geol. Sci. 83: 1–203
MacGinitie, H. D. (1953) Fossil plants of the Florissant Beds, Colorado. Carnegie Institute of Washington, Publication 599, Washington, DC.
McClain A. M. and Manchester S. R. (2001). Dipteronia (Sapindaceae) from the Tertiary of North America and implications for the phytogeographic history of the Aceroideae. Amer. J. Bot. 88: 1316–1325
McLoughlin S. (2001). The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Austral. J. Bot. 49: 271–300
Miceli N., Taviano M. F., Tzakou O., Yannitsaros A., Vassiliades D., Giuffrida D. and Galati E. M. (2005). Biebersteinia orphanidis Boiss. shows antioxidant and anti-inflammatory activity. Phcog. Mag. 1: 54–58
Muellner A. N., Samuel R., Johnson S. A., Cheek M., Pennington T. D. and Chase M. W. (2003). Molecular phylogenetics of Meliaceae based on nuclear and plastid DNA sequences. Amer. J. Bot. 90: 471–480
Muellner A. N., Savolainen V., Samuel R. and Chase M. W. (2006). The mahogany family “out-of-Africa”: divergence time estimation, global biogeographic patterns inferred from plastid rbcL DNA sequences, extant and fossil distribution of diversity. Molec. Phylogenet. Evol. 40: 236–250
Ngamriabsakul C., Newman M. F. and Cronk Q. C. B. (2000). Phylogeny and disjunction in Roscoea (Zingiberaceae). Edinb. J. Bot. 57: 39–61
Olmstead R. G., Michaels H. J., Scott K. M. and Palmer J. D. (1992). Monophyly of the Asteridae and identification of their major lineages inferred from DNA sequences of rbcL. Ann. Missouri Bot. Gard. 79: 249–265
Palmer A. R., Geissman J. (1999) Geologic time scale. The Geological Society of America. Available at: http://www.geosociety.org/science/timescale/timescl.pdf.
Posada D. and Crandall K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817–818
Rambaut A., Charleston M. (2000) Phylogenetic Tree Editor v1.0 alpha 4–61. http://evolve. zoo.ox.ac.uk/software/TreeEdit.
Reid E. M. and Chandler M. E. J. (1933). The London Clay Flora. British Museum (Natural History), London, UK
Ronquist F. (1996) DIVA version 1.1. Computer program and manual available from Uppsala University (http://www.ebc.uu.se/systzoo/research/diva/diva.html).
Ronquist F. (1997). Dispersal-vicariance analysis: a new approach to the quantification of historical biogeography. Syst. Biol. 46: 195–203
Ronquist F. and Huelsenbeck J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574
Salamin N., Chase M. W., Hodkinson T. R. and Savolainen V. (2003). Assessing internal support with large phylogenetic DNA matrices. Molec. Phylogenet. Evol. 27: 528–539
Sanderson M. J. (1997). A nonparametric approach to estimating divergence times in the absence of rate constancy. Molec. Biol. Evol. 14: 1218–1232
Sanderson M. J. (1998). Estimating rate and time in molecular phylogenies: beyond the molecular clock?. In: Soltis, D. E., Soltis, P. S. and Doyle, J. J. (eds) Molecular systematics of plants II: DNA sequencing, pp 242–264. Kluwer, Boston, Massachusetts, USA
Sanderson M. J., Donoghue M. J., Piel W. H. and Eriksson T. (1994). TreeBASE: A prototype database of phylogenetic analyses and an interactive tool for browsing the phylogeny of life. Amer. J. Bot. 81: 183
Sanderson M. J., Thorne J. L., Wikström N. and Bremer K. (2004). Molecular evidence on plant divergence times. Amer. J. Bot. 91: 1656–1665
Schönbeck-Temesy E. (1970). Geraniaceae: Biebersteinia. In: Rechinger, K. H. (eds) Flora Iranica, pp 63–64. Akademische Druck- u. Verlagsanstalt, Graz
Soltis P. S., Soltis D. E., Chase M. W. (1999) Angiosperm phylogeny inferred from multiple genes: a research tool for comparative biology. Nature: 402–404.
Soltis D. E. (2000). Angiosperm phylogeny inferred from a combined dataset of 18S rDNA, rbcL and atpB sequences. Bot. J. Linn. Soc. 133: 381–461
Song Z.-C., Wang W.-M. and Fei H. (2004). Fossil pollen records of extant angiosperms in China. Bot. Rev. 70: 425–458
Stamatakis A. (2006). RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690
Stephan F. (1806). Déscription de deux nouveaux genres de plantes. Mém. Soc. Nat. Mosc. 1: 125–128
Swofford D. L. (2002). PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, Massachusetts, USA
Summerfield M. A. (1991). Global geomorphology. Prentice Hall, USA, 71–72
Takhtajan A. (1986). Floristic regions of the world. University of California Press, Berkeley Los Angeles
Takhtajan A. (1997). Diversity and classification of flowering plants. Columbia University Press, New York
Thorne J. L. and Kishino H (2002). Divergence time estimation and rate evolution with multilocus data sets. Syst. Biol. 51: 689–702
Tzakou O., Yannitsaros A. and Vassiliades D. (2001). Investigation of the C16:3/C18:3 fatty acid balance in leaf tissues of Biebersteinia orphanidis Boiss. (Biebersteiniaceae). Biochem. Syst. Ecol. 29: 765–767
Vassiliades D. and Yannitsaros A. (2000). Orphanides's best discovery. Bot. Chron. 13: 241–248
Vidya T. N. C., Fernando P., Melnick D. J. and Sukumar R. (2005). Population genetic structure and conservation of Asian elephants (Elephas maximus) across India. Animal Conservation 8: 377–388
Weeks A., Daly D. C. and Simpson B. B. (2005). The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraceae) based on nuclear and chloroplast sequence data. Molec. Phylogenet. Evol. 35: 85–101
Wikström N., Savolainen V. and Chase M. W. (2001). Evolution of the angiosperms: calibrating the family tree. Proc. Roy. Soc. Lond. B 268: 2211–2220
Yang Z. (1997). PAML: a program package for phylogenetic analysis by maximum likelihood. Comp. Appl. BioSci. 13: 555–556 http://abacus.gene.ucl.ac.uk/software/paml.html
Zhang X. F., Hu B. L. and Zhou B. N. (1995). Studies on the active constituents of Tibetan herb Biebersteinia heterostemon Maxim. Acta Pharmaceutica Sin. 30: 211–214
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muellner, A., Vassiliades, D. & Renner, S. Placing Biebersteiniaceae, a herbaceous clade of Sapindales, in a temporal and geographic context. Plant Syst. Evol. 266, 233–252 (2007). https://doi.org/10.1007/s00606-007-0546-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-007-0546-x