Abstract
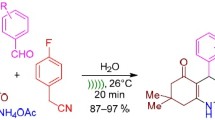
A facile and efficient one-pot, four-component synthesis of polyhydroquinoline derivatives via the Hantzsch reaction using Nafion-H® as heterogeneous catalyst in PEG 400–water solvent system is described herein. The present methodology offers several advantages such as excellent yields, simple procedure, shorter reaction times, and milder conditions with remarkable recyclability.
Graphical Abstract







Similar content being viewed by others
References
Guillena G, Ramon DJ, Yus M (2007) Tetrahedron Asymmetry 18:693
Domling A (2006) Chem Rev 106:17
Simon C, Constantieux T, Rodrigeuz J (2004) Eur J Org Chem 4957
Domling A, Ugi I (2000) Angew Chem Int Ed 39:3168
Ramon DJ, Yus M (2005) Angew Chem Int Ed 44:1602
Mauzerall D, Westheimer FH (1955) J Am Chem Soc 77:2261
Baraldi PG, Budriesi R, Cacciari B, Chiarini A, Garuti L, Giovanninetti G, Leoni A, Roberti M (1992) Collect Czech Chem Commun 57:169
Di Stilo A, Visentin S, Cena C, Gasco AM, Ermondi G, Gasco A (1998) J Med Chem 41:5393
Kawase M, Shah A, Gaveriya H, Motohashi N, Sakagami H, Varga A, Molnar J (2002) Bioorg Med Chem 10:1051
Suarez M, Verdecia Y, Illescas B, Martinez-Alvarez R, Alvarez A, Ochoa E, Seoane C, Kayali N, Martin N (2003) Tetrahedron 59:9179
Shan R, Velazquez C, Knaus EE (2004) J Med Chem 47:254
Bossert F, Meyer H, Wehinger E (1981) Angew Chem Int Ed 20:762
Takenaka T, Usuda S, Nomura T, Maeno H, Sado T (1976) Arzneim Forsch 26:2172
Julius S (1988) J Cardiovasc Pharmacol 12:S27
Liang J-C, Yeh J-L, Wang C-S, Liou S-F, Tsai C-H, Chen I-J (2002) Bioorg Med Chem 10:719
Boschi D, Caron G, Visentin S, Di Stilo A, Rolando B, Fruttero R, Gasco A (2001) Pharm Res 18:987
Godfraind T, Miller R, Wibo M (1986) Pharmacol Rev 38:321
Fleckenstein-Gruen G, Thimm F, Czirfuzs A, Matyas S, Frey M (1994) J Cardiovasc Pharmacol 24:75
Manpadi M, Uglinskii PY, Rastogi SK, Cotter KM, Wong Y-SC, Anderson LA, Ortega AJ, Van Slambrouck S, Steelant WFA, Rogelj S, Tongwa P, Antipin MY, Magedov IV, Kornienko A (2007) Org Biomol Chem 5:3865
Emanuel NM, Obukhova LK, Dubur GJ, Tirzit GD, Uldrikis JR (1985) Dokl Akad Nauk SSSR 284:1271
Bird GLA, Prach AT, McMahon AD, Forrest JAH, Mills PR, Danesh BJ (1998) J Hepatol 28:194
Ogawa AK, Willoughby CA, Bergeron R, Ellsworth KP, Geissler WM, Myers RW, Yao J, Harris G, Chapman KT (2003) Bioorg Med Chem Lett 13:3405
Klusa V (1995) Drugs Future 20:135
Bretzol RG, Bollen CC, Maester E, Federlin KF (1993) Am J Kidney Dis 21:53
Bretzel RG, Bollen CC, Maeser E, Federlin KF (1992) Drugs Future 17:465
Boer R, Gekeler V (1995) Drugs Future 20:499
Eisner U, Kuthan J (1972) Chem Rev 72:1
Stout DM, Meyers AI (1982) Chem Rev 82:223
Kutney JP (1977) Heterocycles 7:593
Widdowson DA (1978) In: Van Tamelen EE (ed) Bioorganic chemistry, Series 4. Academic, New York, p 239
Hantzsch A (1882) Justus Liebigs Ann Chem 215:1
Loev B, Snader KM (1965) J Org Chem 30:1914
Tu S-J, Zhou J-F, Deng X, Cai P-J, Wan H, Feng J-C (2001) Chin J Org Chem 21:313
Sabitha G, Reddy GSKK, Reddy CS, Yadav JS (2003) Tetrahedron Lett 44:4129
Ji S-J, Jiang Z-Q, Lu J, Loh T-P (2004) Synlett 5:831
Breitenbucher JG, Figliozzi G (2000) Tetrahedron Lett 41:4311
Dondoni A, Massi A, Minghini E, Bertolasi V (2004) Tetrahedron 60:2311
Ko S, Sastry MNV, Lin C, Yao C-F (2005) Tetrahedron Lett 46:5771
Ko S, Yao C-F (2006) Tetrahedron 62:7293
Wang L-M, Sheng J, Zhang L, Han J-W, Fan Z-Y, Tian H, Qian C-T (2005) Tetrahedron 61:1539
Maheswara M, Siddaiah V, Damu GLV, Rao CV (2006) Arkivoc 2:201
Heravi MM, Bakhtiari K, Javadi NM, Bamoharram FF, Saeedi M, Oskooie HA (2007) J Mol Catal A Chem 264:50
Nagarapu L, Kumari MD, Kumari NV, Kantevari S (2007) Catal Commun 8:1871
Olah GA, Iyer PS, Prakash GKS (1986) Synthesis 513
Olah GA, Prakash GKS, Sommer J (1985) Superacids. Wiley Interscience, New York
Arata K (1990) Adv Catal 37:165
Yeo SC, Eisenberg A (1977) J Appl Polym Sci 21:875
Yeager HL, Eisenberg A (1982) In: Eisenberg A, Yeager HL (eds), Perflourinated ionomer membranes. ACS Symposium series, vol. 180, American Chemical Society, Washington, DC, p 1
Polshettiwar V, Varma RS (2008) Acc Chem Res 41:629
Polshettiwar V, Varma RS (2008) Chem Soc Rev 37:1546
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, Oxford
Chen J, Spear SK, Huddleston JG, Rogers RD (2005) Green Chem 7:64
Zhang Z-H, Yin L, Wang Y-M, Liu J-Y, Li Y (2004) Green Chem 6:563
Kumar R, Chaudhary P, Nimesh S, Chandra R (2006) Green Chem 8:356
Winter A, van den Berg AMJ, Hoogenboom R, Kickelbick G, Schubert US (2006) Synthesis 2873
Han W, Liu C, Jin Z-L (2007) Org Lett 9:4005
Smith CB, Raston CL, Sobolev AN (2005) Green Chem 7:650
Kidwai M, Bhatnagar D, Chauhan R (2011) J Heterocycl Chem. doi:10.1002/jhet.1037
Kidwai M, Chauhan R, Bhatnagar D (2011) J Sulfur Chem 32:37
Seen AJ (2001) J Mol Catal A Chem 177:105
Kumar S, Sharma P, Kapoor KK, Hundal MS (2008) Tetrahedron 64:536
Mobinikhaledi A, Foroughifar N, Fard MAB, Moghanian H, Ebrahimi S, Kalhor M (2009) Synth Commun 39:1166
Bandgar BP, More PE, Kamble VT, Totre JV (2008) Arkivoc 15:1
Lingaiah BV, Ezikiel G, Yakaiah T, Reddy GV, Rao PS (2006) Synlett 15:2507
Zhu D, Chen J, Xiao H, Liu M, Ding J, Wu H (2009) Synth Commun 39:2895
Sheldrick GM (1997) SHELXL 97, A program for the determination of crystal structure. Anorganisch-Chemisches Institut der Universität Göttingen, Germany
Cromer DT, Mann JB (1968) Acta Cryst A24:321
Stewart RF, Davidson ER, Simpson WT (1965) J Chem Phys 42:3175
Acknowledgments
Ritika Chauhan is grateful to UGC (University Grants Commission) for providing junior research fellowship. We express our thanks to the Director of University Science and Instrumentation Centre, University of Delhi, Delhi, for providing the spectral data and also acknowledge the Head of the Biophysics Department, All India Institute of Medical Sciences, New Delhi, for carrying out the X-ray studies.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kidwai, M., Chauhan, R., Bhatnagar, D. et al. Nafion-H®-catalyzed synthesis of polyhydroquinolines via the Hantzsch multicomponent reaction. Monatsh Chem 143, 1675–1680 (2012). https://doi.org/10.1007/s00706-012-0742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0742-4