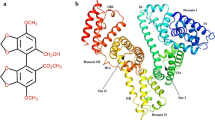
Abstract
The interaction between human serum albumin (HSA) and haloperidol (HPD) was studied by fluorescence and absorption spectroscopy and molecular modeling under physiological conditions. Fluorescence spectroscopic data showed that the fluorescence quenching of HSA was a result of the formation of the HPD–HSA complex. Spectroscopic analysis of the emission quenching at different temperatures revealed that the quenching mechanism of human serum albumin by haloperidol shows a dynamic quenching. The binding constant (K) and the binding sites (n) between haloperidol and HSA were estimated to be 7.94 × 103 dm3 mol−1 and 1.12 at 298 K. The results of thermodynamic parameters, ΔH (− 89.56 kJ mol−1), ΔS (225.94 J mol−1 K−1) and ΔG (− 15.69 kJ mol−1), indicated that the binding process was spontaneous and the van der Waals interactions and hydrogen bonds were the main forces to stabilize the complex. The distance between the donor (HSA) and acceptor (HPD) molecules was obtained according to Förster energy transfer. The effects of metal ions (Ca2+, Mg2+, Cu2+, and Fe3+) on the binding constant of the haloperidol–HSA complex were also investigated. Finally, the binding of haloperidol to HSA was modeled using the molecular docking method. Molecular docking results were in agreement with the experimental conclusions of the thermodynamic parameters.
Graphical abstract











Similar content being viewed by others
References
Petitpas I, Battacharya AA, Twine S, East M, Curry S (2001) J Biol Chem 276:22804
Dugaiczyk A, Law SW, Dennison OE (1982) Proc Natl Acad Sci USA 79:71
Sudlow G, Birkett DJ, Wade DN (1975) Mol Pharmacol 11:824
Sudlow G, Birkett DJ, Wade DN (1976) Mol Pharmacol 12:1052
He XM, Carter DC (1992) Nature 358:209
Curry S, Mandelkow H, Brick P, Franks N (1998) Nat Struct Biol 5:827
Bhattacharya AA, Curry S, Franks NP (2000) J Biol Chem 275:38731
Oida T (1986) J Biochem 100:99
Peters T (1995) All about albumin: biochemistry, genetics, and medical applications. Academic Press, San Diego
Peck ET, Hill S, Williams AM (2008) Pharmacology for anaesthesia and intensive care, 3rd edn. Cambridge University Press, New York
Peluso MJ, Lewis SW, Barnes TR, Jones PB (2012) Br J Psychiatry 200:387
Chien WT, Yip AL (2013) Neuropsychiatr Dis Treat 9:1311
Seeman P, van Tol HHM (1994) Trends Pharmacol Sci 15:264
Lakowicz JR (2006) Principles of fluorescence spectroscopy, 3rd edn. Springer, New York
Carter DC, Ho JX (1994) Adv Protein Chem 45:153
Lakowicz JR, Weber G (1973) Biochemistry 12:4161
Chen GZ, Huang XZ, Xu JG, Zheng ZZ, Wang ZB (1990) The methods of fluorescence analysis, 2nd edn. Science Press, Beijing
Naik KM, Nandibewoor ST (2013) Spectrochim Acta A 105:418
Stojanović SD, Janković SM, Matović ZD, Jakovljević IŽ, Jelić RM (2015) Monatsh Chem 146:399
Ross PD, Subramanian S (1981) Biochemistry 20:3096
Azimi O, Emami Z, Salari H, Chamani J (2011) Molecules 16:9792
Stryer L, Haugland RP (1967) Proc Natl Acad Sci USA 58:719
Stryer L (1978) Ann Rev Biochem 47:819
Förster T (1959) Faraday Soc 27:7
Förster T (1965) In: Sinanoglu O (ed) Modern quantum chemistry. Academic Press, New York
Epps DE, Raub TJ, Caiolfa V, Chiari A, Zamai M (1999) J Pharm Pharmacol 51:41
Valeur B (2001) Molecular fluorescence: principle and applications. Wiley Press, New York
Naik PN, Chimatadar SA, Nandibewoor ST (2010) J Photochem Photobiol B 100:147
Bal W, Sokołowska M, Kurowska E, Faller P (2013) Biochim Biophys Acta 1830:5444
Chikvaidze E (1988) Biofizika 33:723
Seedher N, Agarwal P (2010) Drug Metab Drug Interact 25:17
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) J Comput Chem 30:2785
Trott O, Olson AJ (2010) Comput J Chem 31:455
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) J Mol Biol 267:727
Wang Z, Sun H, Yao X, Li D, Xu L, Li Y, Tiand S, Hou T (2016) Phys Chem Chem Phys 18:12964
Petitpas I, Petersen CE, Ha C, Bhattacharya AA, Zunszain PA, Ghuman J, Bhagavan NV, Curry S (2003) Proc Natl Acad Sci USA 100:6440
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearmark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth G, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 Revision D. Gaussian Inc, Wallingford
Acknowledgements
The authors are grateful to the Ministry of Science and Technological Development of the Republic of Serbia for financial support (Grant nos 172016 and III41010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berić, J.D., Stojanović, S.D., Mrkalić, E.M. et al. Interaction of haloperidol with human serum albumin and effect of metal ions on the binding. Monatsh Chem 149, 2359–2368 (2018). https://doi.org/10.1007/s00706-018-2310-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-018-2310-z