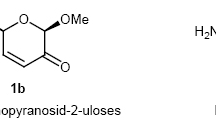
Summary.
D-Glycero-D-gulo-heptose reacted with 2,2-dimethoxypropane to give its 2,3:6,7-di-O-isopropylidene derivative. Its base-catalyzed addition to formaldehyde resulted in the formation of 2,3:6,7-di-O-isopropylidene-2-C-(hydroxymethyl)-D-glycero-D-gulo-heptofuranose. After acid hydrolysis of this aldolization product, a new branched-chain aldose, 2-C-(hydroxymethyl)-D-glycero-D-gulo-heptose, was obtained, which was stereospecifically rearranged under the catalytic action of molybdic acid to D-glycero-D-ido-oct-2-ulose.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received October 17, 2000. Accepted December 4, 2000
Rights and permissions
About this article
Cite this article
Hricovíniová, Z., Hricovíni, M. & Petruš, L. Stereospecific Synthesis of D-Glycero-D-ido-oct-2-ulose. Monatshefte fuer Chemie 132, 731–737 (2001). https://doi.org/10.1007/s007060170088
Issue Date:
DOI: https://doi.org/10.1007/s007060170088