Abstract
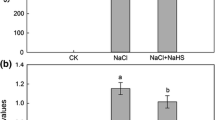
Hydrogen sulfide (H2S) and hydrogen peroxide (H2O2) function as the signaling molecules in plants responding to salt stresses. The present study presents a signaling network involving H2S and H2O2 in salt resistance pathway of the Arabidopsis root. Arabidopsis roots were sensitive to 100 mM NaCl treatment, which displayed a great increase in electrolyte leakage (EL) and Na+/K+ ratio under salt stress. The treatment of H2S donors sodium hydrosulfide (NaHS) enhanced the salt tolerance by maintaining a lower Na+/K+ ratio. In addition, the inhibition of root growth under salt stress was removed by H2S. Further studies indicated that H2O2 was involved in H2S-induced salt tolerance pathway. H2S induced the production of the endogenous H2O2 via regulating the activities of glucose-6-phosphate dehydrogenase (G6PDH) and plasma membrane (PM) NADPH oxidase, with the treatment with dimethylthiourea (DMTU, an ROS scavenger), diphenylene iodonium (DPI, a PM NADPH oxidase inhibitor), or glycerol (G6PDH inhibitor) removing the effect of H2S. Treatment with amiloride (an inhibitor of PM Na+/H+ antiporter) and vanadate (an inhibitor of PM H+-ATPase) also inhibited the activity of H2S on Na+/K+ ratio. Through an analysis of quantitative real-time polymerase chain reaction and Western blot, we found that H2S promoted the genes expression and the phosphorylation level of PM H+-ATPase and Na+/H+ antiporter protein level. However, when the endogenous H2O2 level was inhibited by DPI or DMTU, the effect of H2S on the PM Na+/H+ antiporter system was removed. Taken together, H2S maintains ion homeostasis in the H2O2-dependent manner in salt-stress Arabidopsis root.








Similar content being viewed by others
References
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581:2247–2254
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216:355–365
Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465:140–151
Bradford MM (1976) A rapid and sensitive method for the quantitationof microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122
Chen J, Wang WH, Wu FH, You CY, Liu WT, Dong XK, He JX, Zheng HL (2013) Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362:301–318
Christoul A, Manganaris GA, Papadopoulos I, Fotopoulos V (2013) Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 647:1953–1966
Filippou P, Antoniou C, Fotopoulos V (2011) Effect of drought and rewatering on the cellular status and antioxidant response of Medicago truncatula plants. Plant Signal Behav 6:270–277
Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol 28:89–121
García-Mata C, Lamattina L (2013) Gasotransmitters are emerging as new guard cell signaling molecules and regulators of leaf gas exchange. Plant Sci 201–202:66–73
Hayashi M, Inoue S, Takahashi K, Kinoshita T (2011) Immunohisto-chemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol 52:1238–1248
Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K, Kinoshita T (2010) Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol 51:1186–1196
Hou Z, Wang L, Liu J, Hou L, Liu X (2013) Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J Integr Plant Biol 55:277–289
Hung KT, Hsu YT, Kao CH (2006) Hydrogen peroxide is involved in methyl jasmonate-induced senescence of rice leaves. Plant Physiol 127:293–303
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Levine A, Tenhaken R, Dixon RA, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive response. Cell 79:583–593
Li JS, Wang XM, Zhang YL, Jia HL, Bi YR (2011) cGMP regulates hydrogen peroxide accumulation in calcium-dependent salt resistance pathway in Arabidopsis thaliana roots. Planta 234:709–722
Li ZG, Yang SZ, Long WB, Yang GX, Shen ZZ (2013) Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ 36:1564–1572
Lisjak M, Teklic T, Wilson ID, Whiteman M, Hancock JT (2013) Hydrogen sulfide: environmental factor or signalling molecule? Plant Cell Environ 36:1607–1616
Maffei M, Mithofer A, Arimura G (2006) Effects of feeding Spodoptera littoralis on Lima bean leaves: III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol 140:1022–1035
Matsumura H, Miyachi S (1980) Cycling assay for nicotinamide adenine dinucleotides. Methods Enzymol 69:465–470
Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve ZT (2006) Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato-root-knot nematode interactions. New Phytol 170:501–512
Michelet B, Boutry M (1995) The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108:1–6
Miguel A, Frédéric G, Mohammed O, Marc B (2003) The plasma membrane proton pump ATPase, the significance of gene subfamilies. Planta 216:355–365
Molassiotis A, Fotopoulos V (2011) Oxidative and nitrosative signaling in plants: two branches in the same tree? Plant Signal Behav 6:210–214
Morsomme P, Boutry M (2000) The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim Biophys Acta Biomembranes 1465:1–16
Nashef AS, Osuga DT, Feeney RE (1977) Determination of hydrogen sulfide with 5,5β′-dithiobis-(2-nitrobenzoic acid), N-ethylmaleimide, and parachloromercuribenzoate. Anal Biochem 79:394–405
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:10–12
Niu X, Zhu JK, Narasimhan ML, Bressan RA, Haseqawa PM (1993) Plasma-membrane H+-ATPase gene expression is regulated by NaCl in cells of the halophyte Atriplex nummularia L. Planta 190:433–438
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci U S A 99:8436–8441
Qiu QS, Su XF (1998) The influence of extracellular-side Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots. Aust J Plant Physiol 25:923–928
Rea PA, Poole RJ (1993) Vacuolar H+-translocating pyrophosphatase. Annu Rev Plant Phys 44:157–180
Rea PA, Sander D (1987) Tonoplast energization: two H+ pumps, one membrane. Physiol Plantarum 71:131–141
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in subcellular fraction of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162:897–904
Serrano R, Mulet JM, Rios G (1999) A glimpse of the mechanisms of ion homeostasis during salt stress. J Exp Bot 50:1023–1036
Shan CJ, Zhang SL, Li DF, Zhao YZ, Tian XL, Zhao XL, Wu YX, Wei XY, Liu RQ (2011) Effects of exogenous hydrogen sulfide on the ascorbate and glutathione metabolism in wheat seedlings leaves under water stress. Acta Physiol Plantarum 33:2533–2540
Shi H, Ishitani M, Kim C, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci U S A 97:6896–6901
Sun J, Wang MJ, Ding MQ, Deng SR, Liu MQ, Lu CF, Zhou XY, Shen X, Zheng XJ, Zhang ZK, Song J, Hu MZ, Xu Y, Chen SL (2010) H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ 33:943–958
Tan BH, Wong PTH, Bian JS (2010) Hydrogen sulfide: a novel signaling molecule in the central nervous system. Neurochem Intl 56:3–10
Van Gestelen P, Asard H, Caubergs RJ (1997) Solubilization and separation of a plant plasma membrane NADPH-O2-synthase from other NAD(P)H oxidoreductases. Plant Physiol 115:543–550
Veljovic-Jovanovic SD, Noctor G, Foyer CH (2002) Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem 40:501–507
Wahid A, Perveen M, Gelani S, Basra SMA (2007) Pre-treatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J Plant Physiol 164:283–294
Wang HH, Liang XL, Wan Q, Wang XM, Bi YR (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307
Wang XM, Ma YY, Huang CH, Wan Q, Li N, Bi YR (2008) Glucose-6-phosphate dehydrogenase plays a central role in modulating reduced glutathione levels in reed callus under salt stress. Planta 227:611–623
Wang Y, Li L, Cui W, Xu S, Shen W, Wang R (2012) Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351:107–119
Wilson LG, Bressan RA, Filner P (1978) Light-dependent emission of hydrogen sulfide from plants. Plant Physiol 61:184–189
Yang YL, Zhang F, He WL, Wang XM, Zhang LX (2003) Iron-mediated inhibition of H+-ATPase in plasma membrane vesicles isolated from wheat roots. Cell Mol Life Sci 60:1249–1257
Yu LJ, Lan WZ, Chen C, Yang Y (2004) Glutathione levels control glucose-6-phosphate dehydrogenase activity during elicitor induced oxidative stress in cell suspension cultures of Taxus chinensis. Plant Sci 167:329–335
Zhang F, Wang Y, Yang Y, Wu H, Wang D, Liu J (2007) Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ 30:775–785
Zhang X, Wang, H, Takemiya A, Song CP, Kinoshita T, Shimazaki K (2004) Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol 136:4150–4158
Zhao LQ, Zhang F, Guo JK, Yang YL, Li BB, Zhang LX (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134:849–857
Zhao MG, Tian QY, Zhang WH (2007) Nitric oxide synthase-dependent nitric oxide production is associated with salt tolerance in Arabidopsis. Plant Physiol 144:206–217
Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 4:401–406
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Acknowledgments
Support for this work is from the Northwest A&F University doctor start-up Foundation (Z109021116).
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Jisheng Li and Honglei Jia these two authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, J., Jia, H., Wang, J. et al. Hydrogen sulfide is involved in maintaining ion homeostasis via regulating plasma membrane Na+/H+ antiporter system in the hydrogen peroxide-dependent manner in salt-stress Arabidopsis thaliana root. Protoplasma 251, 899–912 (2014). https://doi.org/10.1007/s00709-013-0592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0592-x