Abstract
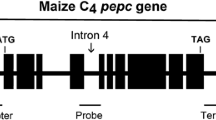
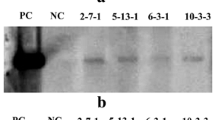
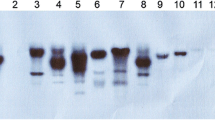
Using particle bombardment transformation, we introduced maize pepc cDNA encoding phosphoenolpyruvate carboxylase (PEPC) and ppdk cDNA encoding pyruvate orthophosphate dikinase (PPDK) into the C3 crop wheat to generate transgenic wheat lines carrying cDNA of pepc (PC lines), ppdk (PK lines) or both (PKC lines). The integration, transcription, and expression of the foreign genes were confirmed by Southern blot, Real-time quantitative reverse transcription PCR (Q-RT–PCR), and Western blot analysis. Q-RT-PCR results indicated that the average relative expression levels of pepc and ppdk in the PKC lines reached 10 and 4.6, respectively, compared to their expressions in untransformed plants (set to 1). The enzyme activities of PEPC and PPDK in the PKC lines were 4.3- and 2.1-fold higher, respectively, than in the untransformed control. The maximum daily net photosynthetic rates of the PKC, PC, and PK lines were enhanced by 26.4, 13.3, and 4.5 %, respectively, whereas the diurnal accumulations of photosynthesis were 21.3, 13.9, and 6.9 %, respectively, higher than in the control. The Fv/Fm of the transgenic plants decreased less than in the control under high temperature and high light conditions (2 weeks after anthesis), suggesting that the transgenic wheat transports more absorbed light energy into a photochemical reaction. The exogenous maize C4-specific pepc gene was more effective than ppdk at improving the photosynthetic performance and yield characteristics of transgenic wheat, while the two genes showed a synergistic effect when they were transformed into the same genetic background, because the PKC lines exhibited improved photosynthetic and physiological traits.







Similar content being viewed by others
References
Alla MMN, Hassan NM (2012) A possible role for C4 photosynthetic enzymes in tolerance of Zea mays to NaCl. Protoplasma 249:1109–1117
Aoyagi K, Bassham JA (1986) Variation in amounts of pyruvate, orthophosphate dikinase, and some other enzymes of the C4 pathway in some wheat species. Plant Physiol 82:96–98
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen XQ, Zhang XD, Liang QR, Zhang LQ, Yang FP, Cao QM (2004) Molecular cloning of maize C4 pepc and GM research in wheat. Chin Sci Bull 49:1976–1982 (in Chinese)
Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980
Demmig B, Winter K, Krüger A, Czygan F (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224
Ding ZS, Zhao M, Jing YX, Li LB, Kuang TY (2007) Effect of overexpression of maize ppc gene on photosynthesis in transgenic rice plants. Acta Agron Sin 33:717–722 (in Chinese)
Edwards GE, Franceschi VR, Voznesenskaya EV (2004) Single-cell C4 photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55:173–196
Fischer RA, Rees D, Sayre KD, Lu Z, Condon AG, Saavedra AL (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38:1467–1475
Fukayama H, Tsuchida H, Agarie S, Nomura M, Onodera H, Ono K, Lee B, Hirose S, Toki S, Ku MS (2001) Significant accumulation of C4-specific pyruvate, orthophosphate dikinase in a C3 plant, rice. Plant Physiol 127:1136–1146
Gehlen J, Panstruga R, Smets H, Merkelbach S, Kleines M, Porsch P, Fladung M, Becker I, Rademacher T, Hausler RE (1996) Effects of altered phosphoenolpyruvate carboxylase activities on transgenic C3 plant Solanum tuberosum. Plant Mol Biol 32:831–848
Genty B, Briantais J, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta Gen Subj 990:87–92
González M, Sanchez R, Cejudo FJ (2003) Abiotic stresses affecting water balance induce phosphoenolpyruvate carboxylase expression in roots of wheat seedlings. Planta 216:985–992
Han LL, Xu WG, Hu L, Li Y, Qi XL, Zhang JH, Zhang HF, Wang YX (2013) Preliminary study on the physiological characteristics of transgenic wheat with maize C4-pepc gene in field conditions. Cereal Res Commun 1–11
Hatch MD (1987) C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta 895:81–106
Häusler RE, Rademacher T, Li J, Lipka V, Fischer KL, Schubert S, Kreuzaler F, Hirsch HJ (2001) Single and double overexpression of C4 cycle genes had differential effects on the pattern of endogenous enzymes, attenuation of photorespiration and on contents of UV protectants in transgenic potato and tobacco plants. J Exp Bot 52(362):1785–1803.
Hibberd JM, Sheehy JE, Langdale JA (2008) Using C4 photosynthesis to increase the yield of rice—rationale and feasibility. Curr Opin Plant Biol 11:228–231
Hu L, Li Y, Xu WG, Zhang QC, Zhang L, Qi XL, Dong HB (2012) Improvement of the photosynthetic characteristics of transgenic wheat plants by transformation with the maize C4 phosphoenolpyruvate carboxylase gene. Plant Breed 131:385–391
Hudspeth RL, Grula JW, Dai Z, Edwards GE, Ku MS (1992) Expression of maize phosphoenolpyruvate carboxylase in transgenic tobacco effects on biochemistry and physiology. Plant Physiol 98:458–464
Ishimaru K, Ichikawa H, Matsuoka M, Ohsugi R (1997) Analysis of a C4 maize pyruvate, orthophosphate dikinase expressed in C3 transgenic Arabidopsis plants. Plant Sci 129:57–64
Jiao D, Huang X, Li X, Chi W, Kuang T, Zhang Q, Ku MS, Cho D (2002) Photosynthetic characteristics and tolerance to photo-oxidation of transgenic rice expressing C4 photosynthesis enzymes. Photosynth Res 72:85–93
Kogami H, Shono M, Koike T, Yanagisawa S, Izui K, Sentoku N, Tanifuji S, Uchimiya H, Toki S (1994) Molecular and physiological evaluation of transgenic tobacco plants expressing a maize phosphoenolpyruvate carboxylase gene under the control of the cauliflower mosaic virus 35S promoter. Transgenic Res 3:287–296
Kropff MJ, Cassman KG, Peng S, Matthews RB, Setter TL (1994) Quantitative understanding of yield potential. In: Breaking the yield barrier. Proceedings of a workshop on rice yield potential in favorable environments. Manila (Philippines): International Rice Research Institute. pp: 21–38
Ku MS, Kano-Murakami Y, Matsuoka M (1996) Evolution and expression of C4 photosynthesis genes. Plant Physiol 111:949
Ku MS, Agarie S, Nomura M, Fukayama H, Tsuchida H, Ono K, Hirose S, Toki S, Miyao M, Matsuoka M (1999) High-level expression of maize phosphoenolpyruvate carboxylase in transgenic rice plants. Nat Biotechnol 17:76–80
Ku MS, Cho D, Ranade U, Hsu TP, Li X, Jiao DM, Ehleringer J, Miyao M, Matsuoka M (2000) Photosynthetic performance of transgenic rice plants overexpressing maize C4 photosynthesis enzymes. Stud Plant Sci 7:193–204
Ku MS, Cho D, Li X, Jiao D, Pinto M, Miyao M, Matsuoka M (2001) Introduction of genes encoding C4 photosynthesis enzymes into rice plants: physiological consequences Novartis Foundation Symposium 236-Rice Biotechnology: Improving Yield, Stress Tolerance and Grain Quality, pp. 100–116. Wiley Online Library
Lara MV, Chuong SD, Akhani H, Andreo CS, Edwards GE (2006) Species having C4 single-cell-type photosynthesis in the Chenopodiaceae family evolved a photosynthetic phosphoenolpyruvate carboxylase like that of Kranz-type C4 species. Plant Physiol 142:673–684
Li Y, Xu WG, Hu L, Zhang L, Qi XL, Zhang QC, Wang GS (2009) Construction of a high-efficient expression vector for maize Phosphoenolpyruvate Carboxylase gene and its transformation in wheat. J Triticeae Crop 29:741–746 (in Chinese)
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 25:402–408
Magnin NC, Cooley BA, Reiskind JB, Bowes G (1997) Regulation and localization of key enzymes during the induction of Kranz-less, C4-type photosynthesis in Hydrilla verticillata. Plant Physiol 115:1681–1689
Matsuoka M, Fukayama H, Tsuchida H, Nomura M, Agarie S, Ku M, Miyao M (2000) How to express some C4 photosynthesis genes at high levels in rice. Stud Plant Sci 7:167–175
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Murata Y (1981) Dependence of potential productivity and efficiency for solar energy utilization on leaf photosynthetic capacity in crop species. Japanese Journal of Crop Science 50:
Murchie EH, Pinto M, Horton P (2009) Agriculture and the new challenges for photosynthesis research. New Phytol 181:532–552
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4326
O'Leary MH (1982) Phosphoenolpyruvate carboxylase: an enzymologist's view. Annu Rev Plant Physiol 33:297–315
Streatfield SJ, Weber A, Kinsman EA, Hausler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flugge U, Chory J (1999) The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell Online 11:1609–1621
Surridge C (2002) Agricultural biotech: the rice squad. Nature 416:576–578
Taniguchi Y, Ohkawa H, Masumoto C, Fukuda T, Tamai T, Lee K, Sudoh S, Tsuchida H, Sasaki H, Fukayama H (2008) Overproduction of C4 photosynthetic enzymes in transgenic rice plants: an approach to introduce the C4-like photosynthetic pathway into rice. J Exp Bot 59:1799–1809
Tolley BJ, Sage TL, Langdale JA, Hibberd JM (2012) Individual maize chromosomes in the C3 plant oat can increase bundle sheath cell size and vein density. Plant Physiol 159:1418–1427
von Caemmerer S, Quick WP, Furbank RT (2012) The development of C4 rice: current progress and future challenges. Science 336:1671–1672
Voznesenskaya EV, Franceschi VR, Kiirats O, Freitag H, Edwards GE (2001) Kranz anatomy is not essential for terrestrial C4 plant photosynthesis. Nature 414:543–546
Wang YM, Xu WG, Hu L, Zhang L, Li Y, Du XH (2012) Expression of maize gene encoding C4-Pyruvate Orthophosphate Dikinase (PPDK) and C4-Phosphoenolpyruvate Carboxylase (PEPC) in transgenic Arabidopsis. Plant Mol Biol Rep 30:1367–1374
Wu Q, Xu WG, Li Y, Qi XL, Hu L, Zhang L, Han LL (2011) Physiological characteristics of photosynthesis in transgenic wheat with maize C4-PEPC gene under field conditions. Acta Agron Sin 37:2046–2052
Xu XL, ZHANG YH, WANG ZM (2003) Effect of heat stress during grain filling on phosphoenolpyruvate carboxylase and ribulose-1,5-bisphosphate carboxylase/oxygenase activities of various green organs in winter wheat. Photosynthetica 42(2):317–320
Yan JY, Liu XZ (1995) Estimation of photosynthetic accumulation amount and analysis of its rules in wheat. Agric Meteorol 1:5–9 (in Chinese)
Zhang BJ, LING LL, Chen QZ, Hua C, Jiao DM (2009) A key limited factor ATP of constructing C4-like rice. Acta Agric Boreali Sin 24:17–22 (in Chinese)
Zhang JH, Xu WG, Wang HW, Li Y, Hu L, Han LL, Zhang HF (2012) Molecular characteristics and photosynthetic property of the transgenic wheat expressing a maize C4-type pepc gene. J Triticeae Crop 32:1043–1048 (in Chinese)
Zheng TC, Zhang XK, Yin GH, Wang LN, Han YL, Chen L, Huang F, Tang JW, Xia XC, He ZH (2011) Genetic gains in grain yield, net photosynthesis and stomatal conductance achieved in Henan Province of China between 1981 and 2008. Field Crop Res 122:225–233
Zhu X, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261
Acknowledgments
The China Agriculture Research System (no. CARS-3-1-9), Genetically Modified Organisms Breeding Major Projects of China (no. 2011ZX08002-003), National Natural Science Foundation of China (no. 31371707), and National Key Technology R & D Program (nos. 2011BAD07B00 and 2011BAD35B03) supported this work.
Conflict of interest
We declare that the submitted manuscript does not contain previously published material, and is not under consideration for publication elsewhere. Each author has made an important scientific contribution to the study and is thoroughly familiar with the primary data. All authors listed have read the complete manuscript and have approved the submission of the paper. The manuscript is a truthful original work without fabrication, fraud, or plagiarism. All authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Zhang, H., Xu, W., Wang, H. et al. Pyramiding expression of maize genes encoding phosphoenolpyruvate carboxylase (PEPC) and pyruvate orthophosphate dikinase (PPDK) synergistically improve the photosynthetic characteristics of transgenic wheat. Protoplasma 251, 1163–1173 (2014). https://doi.org/10.1007/s00709-014-0624-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0624-1