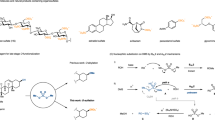
Abstract
The regioselectivity of the ring-opening reactions of 2,2-disubstituted aziridines with sodium bisulfite and sulfite showed significant divergence depending on the nucleophiles and the structure of the substituents of the aziridines. 2,2-Dialkylaziridines were specifically attacked on their less substituted carbon atoms with sodium bisulfite, affording 2,2-substituted taurines, while 2,2-disubstituted aziridines with either one or two aryl substituent(s) were attacked specifically on their more substituted carbon atoms with the same nucleophile, giving rise to aromatic 1,1-substituted taurines. Sodium sulfite attacked the less substituted carbon atoms of all aziridines specifically to afford 2,2-disubstituted taurines. The regioselectivity is governed by the nucleophile and the balance between the steric hindrance and the electronic effect. The current method provides an alternative route to the synthesis of 2,2-dialkyltaurines and aromatic gem-disubstituted taurines.



Similar content being viewed by others
References
Abele S, Seebach D (2000) Preparation of achiral and of enantiopure geminally disubstituted β-amino acids for β-peptide synthesis. Eur J Org Chem 1–15
Alvernhe G, Arsenyiadis S, Chaabouni R, Laurent A (1975) Synthese d’aziridines. Tetrahedron Lett 6:355–356
de Bont DBA, Sliedregt-Bol KM, Hofmeyer LJ F, Liskamp RMJ (1999) Synthesis of phosphono analogues of dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. Bioorg Med Chem 7:1043–1047
Braghiroli D, Di Bella M (1996) New methods for the preparation of 2-amino-2-methylpropanesulfonic acid. Tetrahedron Lett 37:7319–7322
Carson KG, Schwender CF, Shroff HN, Cochran NA, Gallant DL, Briskin MJ (1997) Sulfonopeptide inhibitors of leukocyte adhesion. Bioorg Med Chem Lett 7:711–714
Chen N, Xu JX (2008) An inexpensive, convenient and practical 13C NMR solvent for strong polar amino acid-type compounds. Amino Acids (submitted)
Cordero FM, Cacciarini M, Machetti F, De Sarlo F (2002) Amino-sulfonation of alkenes in a three-component reaction. Eur J Org Chem 1407–1411
Gennari C, Longari C, Ressel S, Salom B, Piarulli U, Ceccarelli U, Mielgo A (1998) Synthesis of combinatorial libraries of vinylogous sulfonamidopeptides. Eur J Org Chem 2437–2449
Gold MH, Skebelsky M, Lang G (1951) The preparation of aliphatic nitro sulfonates. II. β-Amino sulfonic acids. J Org Chem 16:1500–1503
Hu LB, Zhu H, Du DM, Xu JX (2007) Efficient synthesis of taurine and structurally diverse substituted taurines from aziridines. J Org Chem 72:4543–4546
Huang JX, Wang F, Du DM, Xu JX (2005) An expeditious synthesis of 1-substituted and cyclic taurines. Synthesis 2122–2128
Huang JX, Du DM, Xu JX (2006) Facile synthesis of 1,1-disubstituted taurines. Synthesis 315–319
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Juaristi E, Lopez-Ruiz H (1999) Recent advances in the enantioselective synthesis of beta-amino acids. Cure Med Chem 6:983–1004
Koller W, Linkies A, Rehling H, Reuschling D, Hoechst A-G (1983) Synthesis and properties of β-sultams. Tetrahedron Lett 24:2131–2134
Lowik DWPM, Liskamp RMJ (2000) Synthesis of α- and β-substituted aminoethane sulfonamide arginine-glycine mimics. Eur J Org Chem 1219–1228
Ma LG, Xu JX (2004) Nucleophilic ring opening reaction of unsymmetric aziridines and its regioselectivity. Prog Chem 16:220–235
Ohfune Y, Shinada T (2003) Asymmetric Strecker route toward the synthesis of biologically active α,α-disubstituted α-amino acids. Bull Chem Soc Jpn 76:1115–1129
Wang BY, Zhang W, Zhang LL, Du DM, Liu G, Xu JX (2008) Versatile synthesis of free and N-benzyloxycarbonyl-protected 2,2-disubstituted taurines. Eur J Org Chem 350–355
Wirth T (2000) In: Schmalz H.-G (ed) Organic synthesis highlights, vol 4. Wiley, Germany, pp 26–33
Xu JX (2002) A new and expeditious asymmetric synthesis of (R)- and (S)-2-aminoalkanesulfonic acids from chiral amino alcohols. Tetrahedron: Asymmetry 13:1129–1134
Xu JX (2003) Synthesis of hydroxyalkanesulfonic acids, aminoalkanesulfonic acids and sulfonopeptides. Chin J Org Chem 23:1–9
Xu JX, Xu S (2004) A general route to synthesis of N-protected 1-substituted and 1,2-disubstituted taurines. Synthesis 276–279
Xu JX, Xu S (2005) The first synthesis of optically active 1-substituted taurines. Heteroatom Chem 16:466–471
Zhang W, Wang BY, Chen N, Du DM, Xu JX (2008) Expeditious and practical synthesis of various substituted taurines from amino alcohols. Synthesis 197–200
Zhu M, Hu LB, Chen N, Du DM, Xu JX (2008) Synthesis of NH-aziridines from vicinal amino alcohols via the Wenker reaction: scope and limitation. Lett Org Chem 5:212–217
Acknowledgment
The project was supported partly by National Natural Science Foundation of China (Nos. 20472005 and 20772005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, N., Zhu, M., Zhang, W. et al. Synthesis of gem-disubstituted taurines by the regioselective ring-opening of 2,2-disubstituted aziridines with sodium bisulfite and sulfite. Amino Acids 37, 309–313 (2009). https://doi.org/10.1007/s00726-008-0153-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-008-0153-3