Abstract
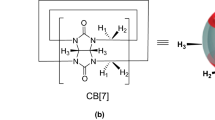
Bent metallocenes (BM) have anti-tumor properties but they face a serious drug efficacy problem due to poor aqueous solubility and rapid hydrolysis under physiological conditions. These two problems can be fixed by encapsulating them in host molecules such as cyclodextrin (CD), cucurbituril (CB) etc. Experimentally, CD-BM, CB-BM host–guest complexes have been investigated to check the efficiency of the drug delivery and efficiency of the encapsulated drug. CB has been reported to be a better host than CD but the reasons for this has not been figured out. This can be done by finding out the mechanism of binding and the nature of the binding forces in both the inclusion complexes. This is exactly done here by performing a DFT study at BP86/TZP level on CB-BM host–guest systems. For comparison CD-BM with β-cyclodextrin as host have been studied. Four BMs (Cp2MCl2, M=Ti, V, Nb, Mo) and their corresponding cations (Cp2MCl+, Cp2M2+) are chosen as guests and they are encapsulated into cucurbit-[6]-uril (CB[6]) and cucurbit-[7]-uril(CB[7]) host systems. Computations reveal that CB[7] accommodates well the BMs over CB[6] due to their larger cavity size and also CB[7] is found to be a better host than β-cyclodextrin. BMs enter vertically rather than horizontally into the CB cavity. The reversible binding of BMs within CB[7] is controlled by various non-bonding interactions and mainly by hydrogen bonding between the portal oxygen atoms and Cp protons as revealed by QTAIM analysis. On the other hand, the interaction between the wall nitrogen atoms in CB[7] and chlorine atoms attached to the metal in BM strengthens the M–Cl bonds that prevents rapid hydrolysis of M–Cl and M–Cp bonds saving the drug. Comparatively, BMs experience less electrostatic attraction and more Pauli repulsion within β-cyclodextrin cavity and this affects the drug binding with CD. This makes β-cyclodextrin a less suitable drug carrier for BMs than CBs. Among the four BMs, niobocene binds strongly and titanocene binds weakly with CBs. EDA clearly shows that all the interactions between the guest and host are non-covalent in nature and electrostatic interactions outperform high-repulsion resulting in stable complexes. Cations form stronger complexes than neutral BMs. FMO analysis reveals that neutral BMs are less reactive compared to their cations and complexes are more reactive in CB[6] environment due to excess strain. QTAIM analysis helps to bring out the newer insights in these types of host–guest systems.










Similar content being viewed by others
References
Gasser G, Ott I, Metzler-Nolte N (2010) J Med Chem 54:3–25
Dowling CM, Claffey J, Cuffe S, Fichtner I, Pampillon C, Sweeney NJ, Strohfeldt K, Watson RG, Tacke M (2008) Lett Drug Des Discov 5:141–144
Hogan M, Cotter J, Claffey J, Gleeson B, Wallis D, O’Shea D, Tacke M (2008) Helv Chim Acta 91:1787–1797
Hogan M, Gleeson B, Tacke M (2010) Lett Drug Des Discov 7:310–317
Strohfeldt K, Tacke M (2008) Chem Soc Rev 37:1174–1187
Pampillón C, Sweeney NJ, Strohfeldt K, Tacke M (2007) J Organomet Chem 692:2153–2159
Senthilnathan D, Vaideeswaran S, Venuvanalingam P (2011) JMol Model 17:465–475
Rahel PE, Yvonne H, Stefan S (2017) CHIMIA Int J Chem 71:120–123. https://doi.org/10.2533/chimia.2017.120
Cini M, Bradshaw TD, Woodward S (2017) Chem Soc Rev 46:1040–1051
Tinoco AD, Saxena M, Sharma S, Noinaj N, Delgado Y (2016) J Am Chem Soc 138:5659–5665
Senthilnathan D, Kalaiselvan A, Vedha SA, Venuvanalingam P (2014) RSC Adv 4:9556–9563
Bonnet G, Kubicki MM, Moise C, Lazzaroni R, Salvadori P, Vitulli G (1992) Organometallics 11:964–967
Collins RA, Russell AF, Scott RTW, Bernardo R, Doremaele G, Berthoud A, Mountford R (2017) Organometallics 36:2167–2181
Musgrave RA, Russell AD, Hayward DW, Whittell GR, Lawrence PG, Gates PJ, Green JC, Manners I (2017) Nat Chem 9:743–750
Erker G (1999) Chem Soc Rev 28:307–314
Erker G, Kehr G, Fröhlich R (2004) J Organomet Chem 689:1402–1412
Gasser G, Metzler-Nolte N (2012) Curr Opin Chem Biol 16:84–91
Green JC (1998) Chem Soc Rev 27:263–272
Erxleben A (2005) Inorg Chem 44:1082–1094
Güette-Fernández JR, Meléndez E, Maldonado-Rojas R, Ortega-Zúñiga C, Olivero-Verbel J, Parés-Matos EI (2017) J Mol Graph Model 75:250–265
Abeysinghe PM, Harding MM (2007) Dalton Trans. 3474–3482. https://doi.org/10.1039/B707440A
Toney JH, Marks TJ (1985) J Am Chem Soc 107:947–953
Murray JH, Harding MM (1994) J Med Chem 37:1936–1941
Ravera M, Cassino C, Monti E, Gariboldi M, Osella D (2005) J Inorg Biochem 99:2264–2269
Loza-Rosas SA, Saxena M, Delgado Y, Gaur K, Pandrala M, Tinoco AD (2017) Metallomics 9:346–356
Kuo LY, Kanatzidis MG, Sabat M, Tipton AL, Marks TJ (1991) J Am Chem Soc 113:9027–9045
Waern JB, Dillon CD, Harding MM (2005) J Med Chem 48:2093–2099
Waern JB, Harding MM (2004) J Organomet Chem 689:4655–4668
Waern JB, Harding MM (2004) Inorg Chem 43:206–213
Mock WL, Shih NY (1986) J Org Chem 51:4440–4446
Garmann D, Warnecke A, Kalayda GV, Kratz F, Jaehde U (2008) J Control Release 131:100–106
Dibama HM, Clarot I, Fontanay S, Salem AB, Mourer M, Finance C, Duval RE, Regnouf-de-Vains JB (2009) Bio Org Med Chem Lett 19:2679–2682
Gasser G, Ott I, Metzler-Nolte N (2011) J Med Chem 54:3–25
Xu F, Li H, Luo YL, Tang W (2017) ACS Appl Mater Interfaces 9:5181–5192
Venkataramanan NS (2017) Suvitha. J Phys Chem B 121:4733–4744
Uekama K, Hirayama F, Irie T (1998) Chem Rev 98:2045–2076
Buck DP, Abeysinghe PM, Cullinane C, Day AI, Collins JC, Harding MM (2008) Dalton Trans. 2328–2334. https://doi.org/10.1039/B718322D
Pan S, Mondal S, Chattaraj PK (2013) New J Chem 37:2492–2499
Barooah N, Kunwar A, Khurana R, Bhasikuttan AC, Mohanty J (2017) Chem Asian J 12:122–131
Abdolmaleki A, Ghasemi F, Ghasemi JB (2017) Chem Biol Drug Des 89:257–268
Ahmed SA, Maity B, Duley SS, Seth D (2017) J Photochem Photobiol B 168:132–141
Rafael RC, Colilla M, Vallet-Regí M (2017) Expert Opin Drug Deliv 14:229–243
Pereira CC, Nolasco M, Braga SS, Paz FAA, Ribeiro-Claro P, Pillinger M, Goncalves IS (2007) Organometallics 26:4220–4956
Metzler-Nolte N (2010) “Biomedical applications of organometal–peptide conjugates” Medicinal organometallic chemistry. Springer, Berlin, pp 195–217
Yang P, Guo M (1999) Coord Chem Rev 185:189–211
Chen X, Zhou L (2010) J Mol Struct THEOCHEM 940:45–49
Mokdsi G, Harding MM (1998) J Organomet Chem 565:29–35
Liu S, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L (2005) J Am Chem Soc 127:15959–15967
Jeon YJ, Kim SY, Ko YH, Sakamoto S, Yamaguchi K, Kim K (2005) Org Biomol Chem 3:2122–2125
Kim J, Ahn Y, Park KM, Kim Y, Ko YH, Oh DH, Kim K (2007) Angew Chem Int Ed 46:7393–7395
Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L (2005) Angew Chem Int Ed 44:4844–4870
Gossens C, Tavernelli I, Rothlisberger U (2005) Chimia Int J Chem 59:81–84
McLaughlin ML, Cronan JM Jr, Schaller TR, Snelling RD (1990) J Am Chem Soc 112:8949–8952
Mokdsi G, Harding MM (2001) J Inorg Biochem 83:205–209
Te Velde G, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, van Gisbergen SJ, Snijders JG, Ziegler T (2001) J Comput Chem 22:931–967
Becke AD (1988) Phys Rev A 38:3098–3100
Perdew JP (1986) Phys Rev B 33:8822–8824
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Valeev EF, Schaefer HF (1998) J Chem Phys 108:7197–7201
van Lenthe E, Ehlers A, Baerends EJ (1999) J Chem Phys 110:8943–8953
Wolff S, Ziegler T, Van Lenthe E, Baerends E (1999) J Chem Phys 110:7689–7698
Morokuma K (1971) J Chem Phys 55:1236–1244
Ziegler T, Rauk A (1977) Theor Chim Acta 46:1–10
Hopffgarten MV, Frenking G (2012) WIRESs Comput Mol Sci 2:43 ((Eds. 121))
Biegler-König F, Schönbohm J (2002) J Comput Chem 23:1489–1494
Vedha SA, Solomon RV, Venuvanalingam P (2013) J Phys Chem A 117:3529–3538
Senthilnathan D, Venuvanalingam P (2011) Eur J Inorg Chem 18:2842–2855
Bader RFW (1998) J Phys Chem A 102:7314–7323
Bader RFW (1985) Acc Chem Res 18:9–15
Bader RFW (2009) J Phys Chem A 113:10391–10396
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2004) Gaussian 03, Revision C02. Gaussian, Inc., Wallingford
Sundararajan M (2013) J Phys Chem B 117:13409–13417
Sundararajan M, Vivek S, Bandyopadhyay T, Ghosh SK (2012) J Phys Chem A 116:4388–4395
Sadhu B, Sundararajan M, Bandyopadhyay T (2016) Inorg Chem 55:598–609
Sundararajan M, Solomon RV, Ghosh SK, Venuvanalingam P (2011) RSC Adv 1:1333–1341
Shahi A, Arunan E (2014) Phys Chem Chem Phys 16:22935–22952
Braga SS, Goncalves IS, Pillinger M, Ribeiro-Claro P, Teixeira-Dias JJC (2001) J Organomet Chem 623:11–16
Braga SS, Marques MM, Sousa JB, Pillinger M, Teixeira-Dias JJC, Goncalves IS (2005) J Organomet Chem 690:2905–2912
Braga SS (2010) Curr Org Chem 14:1356–1361
Pereira CCL, Nolasco M, Braga SS, Almeida Paz FZ, Ribeiro-Claro P, Pillinger M, Goncüalves IS (2007) Organomettalics 26:4220–4228
Riviş A, Hădărugă NG, Gârban Z, Hădărugă DI (2012) Chem Cen J 6:129–139
Saenger W, Jacob J, Gessler K, Steiner T, Hoffmann D, Sanbe H, Koizumi K, Smith SM, Takaha T (1998) Chem Rev 98:1787–1802
Hedges AR (1998) Chem Rev 98:2035–2044
Acknowledgements
P. V. thanks, Council for Scientific and Industrial Research (CSIR), India for the award of EmeritusScientistship (Ref. no. 21(0936)/12/EMR–II). DS acknowledges the support and continuous encouragement from Centre for Research and development, PRIST University, Vallam campus, Thanjavur. RVS acknowledges the support and encouragement from the Department of Chemistry and the management of Madras Christian College (Autonomous), Chennai.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Senthilnathan, D., Solomon, R.V., Kiruthika, S. et al. Are cucurbiturils better drug carriers for bent metallocenes? Insights from theory. J Biol Inorg Chem 23, 413–423 (2018). https://doi.org/10.1007/s00775-018-1547-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-018-1547-7