Abstract.
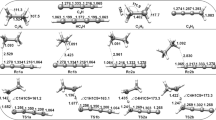
Several new aspects of the O-O bond cleavage and alkane hydroxylation mechanisms have been studied by hybrid density functional theory in this reinvestigation of methane monooxygenase. As concerning key intermediates in these reactions, a new important low-lying state is found, described either as Fe2(III,V) or as Fe2(III,IV)O. A fully optimized transition state for O-O bond cleavage has been determined. It is suggested that the large difference in optimal size (as determined in gas phase) of the complex, before and after the O-O bond cleavage, leads to an additional driving force for the reaction, not considered previously. The strain of the enzyme is estimated to lead to a driving force in the forward direction of about 5 kcal/mol, which could explain some of the pH dependence found in recent experiments. For the hydroxylation reaction, a clean hydrogen abstraction transition state leading to a substrate radical is again found, in contrast to interpretations of radical clock experiments. An explanation, based on new results, is suggested that could account for both the experimental and theoretical results.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Siegbahn, P. O-O bond cleavage and alkane hydroxylation in methane monooxygenase. J. Biol. Inorg. Chem. 6, 27–45 (2001). https://doi.org/10.1007/s007750000184
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s007750000184