Abstract
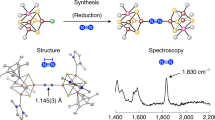
Reactivity studies of clusters that contain the MFe3S4 cores (M = Mo, V) with catecholate, multicarboxylate (or DMF) ligands coordinated to the Mo (or V) atoms, and Cl ligands coordinated to the Fe atoms have been carried out. These studies show the M/Fe/S single cubane clusters to be effective catalysts in the reduction of nitrogenase substrates such as hydrazine, acetylene and protons to give ammonia, ethylene and dihydrogen respectively. The same molecules do not activate or catalyze the reduction of dinitrogen. The results indicate that the observed catalyses are occurring at the Mo (V) sites by a process that, in the case of hydrazine, involves substrate protonation prior to reduction. The facile catalytic reduction of hydrazine by clusters that contain coordinatively saturated polycarboxylate-bound Mo atoms is rationalized in terms of a possible protonation/proton delivery function of the coordinated polycarboxylate ligands. The reactivity characteristics of the M/Fe/S clusters (structurally quite similar to the nitrogenase cofactor) have led to the suggestion that the Mo (V) atoms may be involved in the reduction of hydrazine in the later stages of dinitrogen reduction.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received and accepted: 21 August 1996
Rights and permissions
About this article
Cite this article
Coucouvanis, D. Functional analogs for the reduction of certain nitrogenase substrates. Are multiple sites within the Fe/Mo/S active center involved in the 6e– reduction of N2?. JBIC 1, 594–600 (1996). https://doi.org/10.1007/s007750050098
Issue Date:
DOI: https://doi.org/10.1007/s007750050098