Abstract
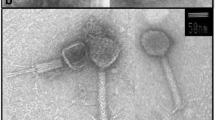
In search for sea ice bacteria and their phages from the Baltic Sea ice, two ice samples were collected from land-fast ice in a south-west Finland coastal site in February and March 2011. Bacteria were isolated from the melted sea ice samples and phages were screened from the same samples for 43 purified isolates. Plaque-producing phages were found for 15 bacterial isolates at 3 °C. Ten phage isolates were successfully plaque purified and eight of them were chosen for particle purification to analyze their morphology and structural proteins. Phage 1/32 infecting an isolate affiliated to phylum Bacteroidetes (Flavobacterium sp.) is a siphovirus and six phages infecting isolates affiliated to γ-Proteobacteria (Shewanella sp.) hosts were myoviruses. Cross titrations between the hosts showed that all studied phages are host specific. Phage solutions, host growth and phage infection were tested in different temperatures revealing phage temperature tolerance up to 45 °C, whereas phage infection was in most of the cases retarded above 15 °C. This study is the first to report isolation and cultivation of ice bacteria and cold-active phages from the Baltic Sea ice.



Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- DGGE:
-
Denaturing gradient gel electrophoresis
- DOM:
-
Dissolved organic matter
- dsDNA:
-
Double-stranded DNA
- PEG:
-
Polyethylene glycol
- Pfu:
-
Plaque forming units
- PVDF:
-
Polyvinylidene difluoride
- SW:
-
South-west
- TEM:
-
Transmission electron microscopy
- TGGE:
-
Temperature gradient gel electrophoresis
References
Atanasova NS, Roine E, Oren A, Bamford DH, Oksanen HM (2012) Global network of specific virus–host interactions in hypersaline environments. Environ Microbiol 14(2):426–440. doi:10.1111/j.1462-2920.2011.02603.x
Avrani S, Wurtzel O, Sharon I, Sorek R, Lindell D (2011) Genomic island variability facilitates Prochlorococcus–virus coexistence. Nature 474(7353):604–608. doi:10.1038/Nature10172
Bohannan BJM, Lenski RE (2000) Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol Lett 3(4):362–377. doi:10.1046/j.1461-0248.2000.00161.x
Boras JA, Sala MM, Arrieta JM, Sa EL, Felipe J, Agusti S, Duarte CM, Vaque D (2010) Effect of ice melting on bacterial carbon fluxes channelled by viruses and protists in the Arctic Ocean. Polar Biol 33(12):1695–1707. doi:10.1007/s00300-010-0798-8
Borriss M, Helmke E, Hanschke R, Schweder T (2003) Isolation and characterization of marine psychrophilic phage–host systems from Arctic sea ice. Extremophiles 7(5):377–384. doi:10.1007/s00792-003-0334-7
Borriss M, Lombardot T, Glockner FO, Becher D, Albrecht D, Schweder T (2007) Genome and proteome characterization of the psychrophilic Flavobacterium bacteriophage 11b. Extremophiles 11(1):95–104. doi:10.1007/s00792-006-0014-5
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 S0003269776699996[pii]
Brinkmeyer R, Knittel K, Jürgens J, Weyland H, Amann R, Helmke E (2003) Diversity and structure of bacterial communities in Arctic versus Antarctic pack ice. Appl Environ Microbiol 69(11):6610–6619
Colangelo-Lillis JR, Deming JW (2013) Genomic analysis of cold-active Colwelliaphage 9A and psychrophilic phage–host interactions. Extremophiles 17(1):99–114. doi:10.1007/s00792-012-0497-1
Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, Kulam-Syed-Mohideen AS, McGarrell DM, Marsh T, Garrity GM, Tiedje JM (2009) The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–D145. doi:10.1093/Nar/Gkn879
Collins RE, Deming JW (2011) Abundant dissolved genetic material in Arctic sea ice part II: viral dynamics during autumn freeze-up. Polar Biol 34(12):1831–1841. doi:10.1007/s00300-011-1008-z
Deming JW (2010) Sea ice bacteria and viruses. In: Thomas DN, Dieckmann GS (eds) Sea ice. Wiley-Blackwell, New York, pp 247–282
Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes—characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17(19):7843–7853. doi:10.1093/nar/17.19.7843
Gowing MM, Garrison DL, Gibson AH, Krupp JM, Jeffries MO, Fritsen CH (2004) Bacterial and viral abundance in Ross Sea summer pack ice communities. Mar Ecol Prog Ser 279:3–12. doi:10.3354/Meps279003
Granskog M, Kaartokallio H, Kuosa H, Thomas DN, Vainio J (2006) Sea ice in the Baltic Sea—a review. Estuar Coast Shelf S 70(1–2):145–160. doi:10.1016/j.ecss.2006.06.001
Haecky P, Jonsson S, Andersson A (1998) Influence of sea ice on the composition of the spring phytoplankton bloom in the northern Baltic Sea. Polar Biol 20(1):1–8. doi:10.1007/s003000050270
Junge K, Imhoff F, Staley T, Deming JW (2002) Phylogenetic diversity of numerically important arctic sea-ice bacteria cultured at subzero temperature. Microb Ecol 43(3):315–328. doi:10.1007/s00248-001-1026-4
Kaartokallio H (2001) Evidence for active microbial nitrogen transformations in sea ice (Gulf of Bothnia, Baltic Sea) in midwinter. Polar Biol 24(1):21–28. doi:10.1007/s003000000169
Kaartokallio H (2004) Food web components, and physical and chemical properties of Baltic Sea ice. Mar Ecol Prog Ser 273:49–63. doi:10.3354/Meps273049
Kaartokallio H, Laamanen M, Sivonen K (2005) Responses of Baltic Sea ice and open-water natural bacterial communities to salinity change. Appl Environ Microb 71(8):4364–4371. doi:10.1128/Aem.71.8.4364-4371.2005
Kaartokallio H, Kuosa H, Thomas DN, Granskog MA, Kivi K (2007) Biomass, composition and activity of organism assemblages along a salinity gradient in sea ice subjected to river discharge in the Baltic Sea. Polar Biol 30(2):183–197. doi:10.1007/s00300-006-0172-z
Kaartokallio H, Tuomainen J, Kuosa H, Kuparinen J, Martikainen PJ, Servomaa K (2008) Succession of sea-ice bacterial communities in the Baltic Sea fast ice. Polar Biol 31(7):783–793. doi:10.1007/s00300-008-0416-1
Kukkaro P, Bamford DH (2009) Virus–host interactions in environments with a wide range of ionic strengths. Environ Microbiol Rep 1(1):71–77. doi:10.1111/j.1758-2229.2008.00007.x
Kuparinen J, Kuosa H, Andersson A, Autio R, Granskog MA, Ikävalko J, Kaartokallio H, Karell K, Leskinen E, Piiparinen J, Rintala JM, Tuomainen J (2007) Role of sea-ice biota in nutrient and organic material cycles in the northern Baltic Sea. Ambio 36(2–3):149–154. doi:10.1579/0044-7447(2007)36[149:Rosbin]2.0.Co;2
Majaneva M, Rintala JM, Piisilä M, Fewer DP, Blomster J (2012) Comparison of wintertime eukaryotic community from sea ice and open water in the Baltic Sea, based on sequencing of the 18S rRNA gene. Polar Biol 35(6):875–889. doi:10.1007/s00300-011-1132-9
Maranger R, Bird DF, Juniper SK (1994) Viral and bacterial dynamics in Arctic sea ice during the spring algal bloom near resolute, N.W.T., Canada. Mar Ecol Prog Ser 111(1–2):121–127. doi:10.3354/Meps111121
Middelboe M, Riemann L, Steward GF, Hansen V, Nybroe O (2003) Virus-induced transfer of organic carbon between marine bacteria in a model community. Aquat Microb Ecol 33(1):1–10. doi:10.3354/Ame033001
Mock T, Thomas DN (2005) Recent advances in sea-ice microbiology. Environ Microbiol 7(5):605–619. doi:10.1111/j.1462-2920.2005.00781.x
Myers EW, Miller W (1988) Optimal alignments in linear space. Comput Appl Biosci 4(1):11–17. doi:10.1093/bioinformatics/4.1.11
Norrman B, Andersson A (1994) Development of ice biota in a temperate sea area (Gulf of Bothnia). Polar Biol 14(8):531–537
Paterson H, Laybourn-Parry J (2012) Antarctic sea ice viral dynamics over an annual cycle. Polar Biol 35(4):491–497. doi:10.1007/s00300-011-1093-z
Petri R, Imhoff JF (2001) Genetic analysis of sea-ice bacterial communities of the Western Baltic Sea using an improved double gradient method. Polar Biol 24(4):252–257. doi:10.1007/s003000000205
Pietilä MK, Laurinmäki P, Russell DA, Ko CC, Jacobs-Sera D, Butcher SJ, Bamford DH, Hendrix RW (2013) Insights into head-tailed viruses infecting extremely halophilic archaea. J Virol 87(6):3248–3260. doi:10.1128/JVI.03397-12
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343:60–62
Rintala JM, Piiparinen J, Ehn J, Autio R, Kuosa H (2006) Changes in phytoplankton biomass and nutrient quantities in sea ice as responses to light/dark manipulations during different phases of the Baltic winter 2003. Hydrobiologia 554:11–24. doi:10.1007/s10750-005-1002-y
Rintala JM, Piiparinen J, Uusikivi J (2010) Drift-ice and under-ice water communities in the Gulf of Bothnia (Baltic Sea). Polar Biol 33(2):179–191. doi:10.1007/s00300-009-0695-1
Staden R, Judge DP, Bonfield JK (2003) Managing sequencing projects in the GAP4 environment. In: Womble DD, Krawetz SA (eds) Introduction to bioinformatics: a theoretical and practical approach. Humana Press, New York
Stedmon CA, Thomas DN, Granskog M, Kaartokallio H, Papadimitriou S, Kuosa H (2007) Characteristics of dissolved organic matter in Baltic coastal sea ice: allochthonous or autochthonous origins? Environ Sci Technol 41(21):7273–7279. doi:10.1021/Es071210f
Sullivan MB, Waterbury JB, Chisholm SW (2003) Cyanophages infecting the oceanic cyanobacterium Prochlorococcus (vol 424, pg 1047. Nature 426(6966):584. doi:10.1038/Nature02147
Suttle CA (2005) Viruses in the sea. Nature 437(7057):356–361. doi:10.1038/nature04160
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naϊve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267. doi:10.1128/AEM.00062-07
Weinbauer MG (2004) Ecology of prokaryotic viruses. FEMS Microbiol Rev 28(2):127–181. doi:10.1016/j.femsre.2003.08.001
Wells LE, Deming JW (2006) Characterization of a cold-active bacteriophage on two psychrophilic marine hosts. Aquat Microb Ecol 45(1):15–29. doi:10.3354/Ame045015
Yager PL, Connelly TL, Mortazavi B, Wommack KE, Bano N, Bauer JE, Opsahl S, Hollibaugh JT (2001) Dynamic bacterial and viral response to an algal bloom at subzero temperatures. Limnol Oceanogr 46(4):790–801
Yamamoto KR, Alberts BM, Benzinge R, Lawhorne L, Treiber G (1970) Rapid bacteriophage sedimentation in presence of polyethylene glycol and its application to large-scale virus purification. Virology 40(3):734. doi:10.1016/0042-6822(70)90218-7
ZoBell CE (1946) Marine microbiology. A monograph on hydrobacteriology. Chronica Botanica Company, Waltham
Acknowledgments
We thank Sari Korhonen for technical assistance. This study was funded by Onni Talas foundation and Walter and Andrée de Nottbeck foundation (AML, JMR) and University of Helsinki three-year grant 2010–2012 (ER). All the field work was carried out at Tvärminne Zoological Station, University of Helsinki whilst the office and laboratory facilities for the isolation work were provided by The Finnish Environmental Institute (SYKE), Marine Research Centre. We thank Academy of Finland (grant 271413) and University of Helsinki for the support to EU ESFRI Instruct Centre for Virus Production and Purification used in this study. This study is dedicated to the memory of Prof. Kielo Haahtela.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Huang.
Rights and permissions
About this article
Cite this article
Luhtanen, AM., Eronen-Rasimus, E., Kaartokallio, H. et al. Isolation and characterization of phage–host systems from the Baltic Sea ice. Extremophiles 18, 121–130 (2014). https://doi.org/10.1007/s00792-013-0604-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0604-y