Abstract
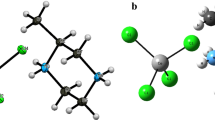
The triazole compound, 5-benzyl-4-(3,4-dimethoxyphenethyl)-2H-1,2,4-triazol-3(4H)-one, has been synthesized and characterized by 1H-NMR, 13C-NMR, IR, and X-ray single-crystal determination. The compound crystallizes in the monoclinic space group P21 with a = 11.8844(3) Å, b = 17.5087(4) Å, c = 17.3648(6) Å, β = 99.990(2)˚ and Z = 8. In addition to the molecular geometry from X-ray experiment, the molecular geometry, vibrational frequencies and gauge including atomic orbital (GIAO) 1H- and 13C-NMR chemical shift values of the title compound in the ground state have been calculated using the density functional method (B3LYP) with 6-31G(d,p) basis set. The calculated results show that the optimized geometries can well reproduce the crystal structure and the theoretical vibrational frequencies and chemical shift values show good agreement with experimental ones. Besides, molecular electrostatic potential (MEP), natural bond orbital (NBO), and frontier molecular orbitals (FMO) analysis of the title compound were performed by the B3LYP/6-31G(d,p) method.







Similar content being viewed by others
References
Zhang Y, Guo ZJ, You XZ (2001) J Am Chem Soc 123:9378–9387
Proft FD, Geerlings P (2001) Chem Rev 101:1451–1464
Fitzgerald G, Andzelm J (1991) J Phys Chem 95:10531–0534
Ziegler T (1991) Pure Appl Chem 63:873–878
Andzelm J, Wimmer E (1992) J Chem Phys 96:1280–1303
Scuseria GE (1992) J Chem Phys 97:7528–7530
Dickson RM, Becke AD (1993) J Chem Phys 99:3898–3905
Johnson BG, Gill PMW, Pople JA (1993) J Chem Phys 98:5612–5626
Oliphant N, Bartlett RJ (1994) J Chem Phys 100:6550–6561
Bhat AR, Bhat GV, Shenoy GG (2001) J Pharm Pharmacol 53:267–272
Al-Soud Yassen A, Al-Dweri Mohammad N, Al-Masoudi Najim A (2004) Il Farmaco 59:775–783
Demirbas N, Ugurluoglu R, Demirbas A (2002) Bioorg Med Chem 10:3717–3723
Emilsson H, Salender H, Gaarder J (1985) Eur J Med Chim Ther 21:333–338
Tozkoparan B, Gökhan N, Aktay G, Yesilada E, Ertan M (2000) Eur J Med Chem 35:743–750
Turan-Zitoui G, Kaplancikli ZA, Erol K, Kilic FS (1999) Il Farmaco 54:218–223
Katica CR, Vesna D, Vlado K, Dora GM, Aleksandra B (2001) Molecules 6:815–824
Kaszuwara W, Leonowicz M, Ukasiewicz A (1992) Mater Lett 12:429–433
Prins R, Biagini-Cingi M, de Graaff RAG, Haasnoot J, Manotti-Lanfredi AM, Rabu P, Reedijk J, Ugozzoli F (1996) Inorg Chim Acta 248:35–44
Drabent K, Biaoska A, Ciunik Z (2004) Inorg Chem Commun 7:224–227
Lyakhov AS, Vorobiov AN, Gaponik PN, Ivashkevich LS, Matulis VE, Ivashkevich OA (2003) Acta Cryst C 59:o690–693
Jia LH, Liu ZL, Liu W (2007) Acta Cryst E 63:o2766
Sorescu DC, Bennett CM, Thompson DL (1998) J Phys A 102:10348–10357
Palmer MH, Christem D (2004) J Mol Struct 705:177–187
Jimenez V, Alderete JB (2006) J Mol Struct (Theochem) 775:1–7
El-Azhary AA, Suter HU, Kubelka J (1998) J Phys Chem A 102:620–629
Da Silva G, Moore EE, Bozzelli JW (2006) J Phys Chem A 110:13979–13988
Matulis VE, Ivashkevich OA, Gaponik PN, Elkind PD, Sukhanov GT, Bazyleva AB, Zaitsau DH (2008) J Mol Struct (Theochem) 854:18–25
Billes F, Endredi H, Keresztury G (2000) J Mol Struct (Theochem) 530:183–200
Krishnakumar V, Xavier RJ (2004) Spectrochim Acta A 60:709–714
Zaza S, Guedira F, Zaydoun S, Saidi Idrissi M, Lautie A, Romain F (2004) Can J Anal Sci Spectrosc 49:15–23
Krishnakumar V, Keresztury G, Sundius T, Xavier RJ (2005) Spectrochim Acta A 61:261–267
Pagacz-Kostrzewa M, Bronisz R, Wierzejewska M (2009) Chem Phys Lett 473:238–246
Pitucha M, Borowski P, Karczmarzyk Z, Fruzinski A (2009) J Mol Struct 919:170–177
Sanchez-Soto PJ, Morillo E, Perez-Rodriguez JL, Real C (1995) J Therm Anal 45:1189–1197
Li J, Litzinger TA (2007) Thermochim Acta 454:116–127
Badea M, Olar R, Marinescu D, Vasile G (2008) J Therm Anal Calorim 92:209–214
Kumar NV, Mashelkar UC (2007) Heterocycl Commun 13:211
Perez-Castro I, Caamano O, Fernandez F, Garcia MD, Lopez C, De Clercq E (2007) Org Biomol Chem 5:3805–3813
Bekirarcan O, Bektas H (2006) Molecules 11:469–477
Haasnoot JG (2000) Coord Chem Rev 131:200–202
Li W, Jia HP, Ju ZF, Zhang J (2006) Cryst Growth Des 6:2136–2140
Chen Z, Li X, Liang F (2008) J Solid State Chem 181:2078–2086
Lin YY, Zhang YB, Zhang JP, Chen XM (2008) Cryst Growth Des 8:3673–3679
Van Koningsbruggen PJ (2004) Top Curr Chem 233:123–149
Bronisz R (2005) Inorg Chem 44:4463–4465
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03 Revision E01. Gaussian Inc, Wallingford, CT
Merrick JP, Moran D, Radom L (2007) J Phys Chem A 111:11683–11700
Frisch A, Dennington R II, Keith T, Millam J, Nielsen AB, Holder AJ, Hiscocks J (2007) GaussView Reference Version 40. Gaussian Inc, Pittsburgh
Ditchfield R (1972) J Chem Phys 56(11):5688–5691
Wolinski K, Hinton JF, Pulay P (1990) J Am Chem Soc 112(23):8251–8260
Politzer P, Murray J (2002) Theor Chem Acc 108:134–142
Farrugia LJ (1997) J Appl Crystallogr 30:565
Hanif M, Qadeer G, Rama NH, Akhtar J, Helliwell M (2009) Acta Cryst E65:o387
Köysal Y, Işık Ş, Doğdaş E, Tozkoparan B, Ertan M (2004) Acta Cryst C60:o356–357
Sancak K, Ustabaş R, Çoruh U, Er M, Ünver Y, Yavuz M (2005) Acta Cryst E61:o1764–1766
Ünver Y, Ustabaş R, Çoruh U, Sancak K, Vazquez-Lopez EM (2006) Acta Cryst E62:o3938–3939
Zhao PS, Xu JM, Zhang WG, Jian FF, Zhang L (2007) Struct Chem 18:993–1000
Genç S, Dege N, Çetin A, Cansız A, Şekerci M, Dinçer M (2004) Acta Cryst E60:o1339–1341
Dinçer M, Avcı D, Şekerci M, Atalay Y (2008) J Mol Model 14:823–832
Vainilavicius P, Smicius R, Jakubkiene V, Tumkevicius S (2001) Manatshefte für Chemie 132:825–831
Scrocco E, Tomasi J (1978) Adv Quantum Chem 11:115–121
Luque FJ, Lopez JM, Orozco M (2000) Theor Chem Acc 103:343–345
Okulik N, Jubert AH (2005) Internet Electron J Mol Des 4:17–30
Politzer P, Laurence PR, Jayasuriya K, McKinney J (1985) Special issue of Environ Health Perspect 61:191–202
Scrocco E, Tomasi J (1973) Topics in current chemistry, vol 7. Springer, Berlin, p 95
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Plenum, New York
Fleming I (1976) Frontier orbitals and organic chemical reactions. Wiley, London
Acknowledgments
This study was supported financially by the Research Centre of Ondokuz Mayıs University (Project No: F-476).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanak, H., Köysal, Y., Yavuz, M. et al. Experimental and DFT computational studies on 5-benzyl-4-(3,4-dimethoxyphenethyl)-2H-1,2,4-triazol-3(4H)-one. J Mol Model 16, 447–457 (2010). https://doi.org/10.1007/s00894-009-0559-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0559-1