Abstract
Hydrogen bonding among hard–hard segments and hard–soft segments in 4,4′-diphenylmethane diisocyanate (MDI)-based polyurethane was investigated theoretically by density functional theory (DFT). Both B3LYP/6-31G* and B3PW91/6-31G* methods gave good structures, reasonable Mulliken charges, binding energies, dipole moments, and good infrared (IR) spectra trends in predicting hydrogen bonding. Bond distances R(N–H⋯O), which were in the range of 3.005–3.028 Å for the carbonyl bonded hydrogen-bond, and 3.074–3.075 Å for the ester bonded hydrogen-bond, are in reasonable agreement with experimental values. Most of the carbonyl oxygen in polyurethane exists in a hydrogen-bonded form. Complex (c), with two carbonyl hydrogen bonds, features the largest dipole moment, while complex (d) with two ester hydrogen bonds, possesses the smallest dipole moment, i.e., lower than that of the isolated monomer, which may be due to the symmetry of the two monomers. These results confirm that the DFT method is a good tool with which to study weak interactions, and indicate that hydrogen bonds are indeed formed between carbonyl and N-H, or ester and N-H, with the former being stronger.

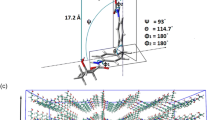
Possible interactions in MDI-based polyurethane.




Similar content being viewed by others
References
Lendlein A, Kelch S (2002) Angew Chem Int Ed 41:2034–2057
Lee BS, Chun BC, Chung YC, Sul KI, Cho JW (2001) Macromolecules 34:6431–6437
Lai YC, Quinn ET (1995) J Polym Sci A 33(11):1767–1772
Takahashi T, Hayashi N, Hayashi S (1996) J Appl Polym Sci 60:1061–1069
Yang JH, Chun BC, Chung YC, Cho JH (2003) Polymer 44:3251–3258
Coleman MM, Skrovanek DJ, Hu J, Painter PC (1988) Macromolecules 21:59–65
Srichatrapimuk VW, Cooper SL (1978) J Macromol Sci Phys 15:267–311
Senich GA, MacKnight WJ (1980) Macromolecules 13:106–110
Brunette CM, Hsu SL, MacKnight WJ (1982) Macromolecules 15:71–77
Christenson CP, Harthcock MA, Meadows MD, Spell HL, Howard WL, Creswick MW, Guerra RE, Turner RBP (1986) J Polym Sci B 24:1401–1439
Lee HS, Wang YK, Hsu SL (1987) Macromolecules 20:2089–2095
Lee SS, Wang YK, MacKnight WI, Hsu SL (1988) Macromolecules 21(1):270–273
Hadzi D (ed) (1997) Theoretical treatment of hydrogen bonding. Wiley, Chichester
Bene JED (1998) Hydrogen bonding 1: encyclopedia of computational chemistry, vol 2. Wiley, New York
Becke AD (1993) J Chem Phys 98:5648–5652
Becke AD (1988) Phys Rev A 38:3098–3100
Lee WYC, Parr RG (1988) Phys Rev B 37:785–789
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6681
Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA (2003) Gaussian 2003W Revision B.05. Gaussian Inc, Pittsburgh
Lord RC, Merrifield RE (1953) J Chem Phys 21:166–167
Teo LS, Chen CY, Kuo JF (1997) Macromolecules 30(6):1793–1799
Vergenz RA, Yazji I, Whittington C, Daw J, Tran KT (2003) J Am Chem Soc 125:12318–12327
Hwang KKS, Wu G, Lin SB, Cooper SL (1984) J Polym Sci Polym Chem Ed 22(7):1677–1697
Luo N, Wang DN, Ying SK (1997) Macromolecules 30(15):4405–4409
Acknowledgment
This work was financially supported by the project “Development of Shape Memory Knitted Fabrics/Garments” (K.14.37.ZR01). The authors wish to express their gratitude for this generous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Hu, J., Chen, S. et al. Theoretical study of hydrogen bonding interactions on MDI-based polyurethane. J Mol Model 16, 1391–1399 (2010). https://doi.org/10.1007/s00894-010-0645-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0645-4