Abstract
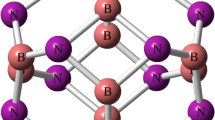
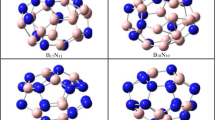
The stability, geometry and electronic structure of the title nanoclusters were compared by using density functional theory (DFT) calculations. Their electrical property analysis showed that the relative magnitude of the HOMO-LUMO gaps (eV) that are average values from the calculated results with five different DFT functionals is as follows:
Computing the standard enthalpy and the Gibbs free energy of formation, it was found that the B12N12 structure is thermodynamically stable at 298 K and 1 atmosphere of pressure, while the Al12N12 structure may be stable at low temperatures. Due to positive values of change of enthalpy and entropy of formation for both the B12P12 and Al12P12 clusters, it seems that their formation from the consisting atoms is not spontaneous at any temperature.

Similar content being viewed by others
References
Kroto H, Heath J, O’Brien S, Curl R, Smalley R (1985) C60: Buckminsterfullerene. Nature 318:162–163
Goel S, Masunov (2011) A density functional theory study of small nickel clusters. J Mol Model. doi:101007/s00894-011-1100-x
Wu HS, Cui XY, Qin XF, Strout D, Jiao H (2006) Boron nitride cages from B12N12 to B36N36: square-hexagon alternants vs boron nitride tubes. J Mol Model 12:537–542. doi:101007/s00894-005-0042-6
Xia Q-Y, Lin Q-F, Zhao W (2011) Theoretical study on the structural, vibrational, and thermodynamic properties ofthe (Br2GaN3)n (n = 1–4) clusters. J Mol Model. doi:101007/s00894-011-1126-0
Yin B, Wang G, Sa N, Huang Y (2008) Bonding analysis and stability on alternant B16N16 cage and its dimmers. J Mol Model 14:789–795. doi:101007/s00894-008-0303-2
Kandalam AK, Blanco MA, Pandey R (2002) Theoretical study of AlnNn, GanNn, and InnNn (n = 4, 5, 6) clusters. J Phys Chem B 106:1945–1953. doi:101021/jp0140062
Costales A, Kandalam AK, Franco R, Pandey R (2002) Theoretical study of structural and vibrational properties of (AlP)n, (AlAs)n, (GaP)n, (GaAs)n, (InP)n, and (InAs)n clusters with n = 1, 2, 3. J Phys Chem B 106:1940–1944. doi:101021/jp013906f
Strout DL (2000) Structure and stability of boron nitrides: isomers of B12N12. J Phys Chem A 104:3364–3366
Wang R, Zhang D, Liu C (2005) Theoretical prediction of a novel inorganic fullerene-like family of silicon-carbon materials. Chem Phys Lett 411:333–338
Oku T, Nishiwaki A, Narita I Formation and atomic structure of B12N12 nanocage clusters studied by mass spectrometry and cluster calculation. Sci Tech Adv Mater 5:635–638
Seifert G, Fowler PW, Mitchell D, Porezag D, Frauenheim T (1997) Boron-nitrogen analogues of the fullerenes: electronic and structural properties. Chem Phys Lett 268:352–358
Jensen F, Toftlund H (1993) Structure and stability of C24 and B12N12 isomers. Chem Phys Lett 201:89–96
Wang Q, Sun Q, Jena P, Kawazoe Y (2009) Potential of AlN nanostructures as hydrogen storage materials. ACS Nano 3:621–626
Feng PY, Balasubramanian K (1999) Spectroscopic properties of Al2P2, Al2P +2 , and Al2P -2 and comparison with their Ga and in analogues. J Phys Chem A 103:9093–9099
Archibong EF, Gregorius RM, Alexander SA (2000) Structures and electron detachment energies of AlP -2 and Al2P2. Chem Phys Lett 321:253–261
Feng PY, Balasubramanian K (2000) Potential energy surfaces of electronic states of AlP2, Al2P and their ions. Chem Phys Lett 318:417–426
Pestana DC, Power PP (1991) Nature of the boron-phosphorus bond in monomeric phosphinoboranes and related compounds. J Am Chem Soc 113:8426–8437
Ferreira V, Alves H (2008) Boron phosphide as the buffer-layer for the epitaxial III-nitride growth: a theoretical study. J Crys Grow 310:3973–3978
Silaghi-Dumitrescu I, Lara-Ochoa F, Haiduc I (1996) A12B12 (A = B, Al; B = N, P) fullerene-like cages and their hydrogenated forms stabilized by exohedral bonds An AM1 molecular orbital study. J Mol Struct THEOCHEM 370:17–23
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100
Ahmadi A, Beheshtian J, Hadipour N (2011) Interaction of NH3 with aluminum nitride nanotube: electrostatic vs. covalent. Physica E 43:1717–1719
Ahmadi A, Kamfiroozi M, Beheshtian J, Hadipour N (2011) The effect of surface curvature of aluminum nitride nanotubes on the adsorption of NH3. Struct Chem. doi:10.1007/s11224-011-9820-1
Beheshtian J, Kamfiroozi M, Bagheri Z, Ahmadi A (2011) Computational study of CO and NO adsorption on magnesium oxide nanotubes. Phys E. doi:10.1016/j.physe.2011.09.016
Ahmadi A, Beheshtian J, Kamfiroozi M Benchmarking of ONIOM method for the study of NH3 dissociation at open ends of BNNTs. J Mol Model. doi:10.1007/s00894-011-1202-5
Hu Y, Ruckenstein E (2003) Ab initio quantum chemical calculations for fullerene cages with large holes. J Chem Phys 119:10073–10080
Ahmadi A, Beheshtian J, Hadipour N (2011) Chemisorption of NH3 at the open ends of boron nitride nanotubes: a DFT study. Struct Chem 22:183–188
Wu H-S, Xu X-H, Strout D, Jiao H (2005) The structure and stability of B36N36 cages: a computational study. J Mol Model 12:1–8
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Hoe W, Cohen A, Handy N (2001) Assessment of a new local exchange functional OPTX. Chem Phys Lett 341:319–328
Xu X, Goddard W (2004) The X3LYP extended density functional for accurate descriptions of nonbond interactions, spin states, and thermochemical properties. Proc Natl Acad Sci USA 101:2673–2677
Zhao Y, Schultz NE, Truhlar DG (2005) Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics, and noncovalent interactions. J Phys Chem 123:161103–161106
Zhao Y, Truhlar DG (2008) Exploring the limit of accuracy of the global hybrid meta density functional for main-group thermochemistry, kinetics, and noncovalent interactions. J Chem Theor Comput 4:1849–1868
Jiling L et al (2007) Theoretical prediction for the (AlN) 12 fullerene-like cage-based nanomaterials. J Phys Cond Matt 19:346228–346236
Yoshida M, Aihara J (1999) Validity of the weighted HOMO-LUMO energy separation as an index of kinetic stability for fullerenes with up to 120 carbon atoms. Phys Chem Chem Phys 1:227–230
Mizorogi N, J-i A (2003) PM3 localization energies for the isolated-pentagon isomers of the C84 fullerene. Phys Chem Chem Phys 5:3368–3371
Chase MW, Davies CA Jr, Downey JR, Frurip DJ Jr, McDonald RA, Syverud AN (1985) J Phys Chem Ref Data 14, Suppl No 1
Ruscic B, Mayhew C, Berkowitz J (1988) Photoionization studies of (BH3)n (n = 1,2). J Chem Phys 88:5580–5593
Storms E, Mueller B (1977) Phase relations and thermodynamic properties of transition metal borides I The molybdenum-boron system and elemental boron. J Phys Chem 81:318–324
Curtiss LA, Raghavachari K, Deutsch PW, Pople J (1991) Theoretical study of Si2Hn (n = 0–6) and Si2H +n (n = 0–7): Appearance potentials, ionization potentials, and enthalpies of formation. J Chem Phys 95:2433–2444
Curtiss LA, Raghavachari K, Redfern PC, Pople J (1997) Assessment of Gaussian-2 and density functional theories for the computation of enthalpies of formation. J Chem Phys 106:1063–1079
Miller JN, Miller JC (2005) Statistics and chemometrics for analytical chemistry, 5th edn. Pearson, Harlow, UK
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beheshtian, J., Bagheri, Z., Kamfiroozi, M. et al. A comparative study on the B12N12, Al12N12, B12P12 and Al12P12 fullerene-like cages. J Mol Model 18, 2653–2658 (2012). https://doi.org/10.1007/s00894-011-1286-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-011-1286-y