Abstract
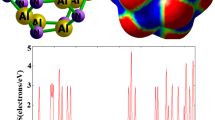
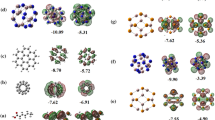
Drug delivery clusters based on nanocages recently have been the most capable to study. Adipic acid (ADPA) interaction mechanism over nanocages of X(Al/B)12Y(N/P)12 was investigated. We analyzed various electronic, chemical, and spectroscopic properties with nanocages of the adsorbed ADPA molecule. Adsorption energies were calculated to study the adsorption of ADPA with nanocages. Raman enhanced surface scattering is used to track the drug as an effective approach to vibrational spectroscopy. Detection of the drug has been investigated using the SERS properties of nanocages. Title drug acts as a donor of electrons and adsorbs at the electrophilic site of nanocages. Variations in chemical descriptors to recognize the sensing property of ADPA-nanocages are also noted. Analysis of various properties explains enhancement which makes it possible to detect the drug in other products.
Graphical abstract

• Interaction of adipic acid with fullerene-like metal nanocages
• Enhancement of spectral properties
• Changes in charge transfer values in nanocage-drug system
• Docking studies identify the drug delivery property




Similar content being viewed by others
Data availability
Data is available on request to the authors.
Code availability
The calculations have been carried out using Gaussian09 and Gaussview version provided by Gaussian Inc.
References
Hamers RJ (2008) Formation and characterization of organic monolayers on semiconductor surface. Annu Rev Anal Chem 1:707–736. https://doi.org/10.1146/annurev.anchem.1.031207.112916
Tao F, Bernasek SL, Xu GQ (2009) Electronic and structural factors in modification and functionalization of clean and passivated semiconductor surface with aromatic systems. Chem Rev 109:3991–4024. https://doi.org/10.1021/cr8003532
Bent SF (2002) Organic functionalization of group IV semiconductor surfaces: principles, examples, applications and prospects. Surf Sci 500:879–903. https://doi.org/10.1016/S0039-6028(01)01553-9
Wang GT, Mui C, Tannaci JF, Filler MA, Musgrave CB, Bent SF (2003) Reactions of cyclic aliphatic and aromatic amines on Ge(100)-2×1 and Si(100)-2×1. J Chem Phys B 107:4982–4996. https://doi.org/10.1021/jp026864j
Kim DH, Choi DS, Hong S, Kim S (2008) Atomic and electronic structure of pyrrole on Ge(100). J Phys Chem C 112:7412–7419. https://doi.org/10.1021/jp709740n
Tian WP, Ji F, Zhang H (2005) Production consumption and development of adipic acid at home and abroad. Chem Int 3:1–4
Haillot D, Bauer T, Kroner U, Tamme R (2011) Thermal analysis of phase change materials in the temperature range 120-150°C. Thermochim Acta 512:49–59. https://doi.org/10.1016/j.tca.2010.11.011
Pielichowska K, Pielichowski K (2014) Phase change materials for thermal energy storage. Prog Mater Sci 65:67–123. https://doi.org/10.1016/j.pmatsci.2014.03.005
Zhou D, Zhao CY, Tian Y (2012) Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl Energy 95:593–605. https://doi.org/10.1016/j.apenergy.2011.08.025
Zhou W, Wei J, Zhu J, Li K, Cheng X (2019) Effect of Dy2O3 on thermal properties of adipic acid (AA) as phase-change materials. J Therm Anal Calorim 138:2999–3005. https://doi.org/10.1007/s10973-019-08315-4
Zhou W, Li K, Zhu J, Li R, Cheng X, Liu F (2017) Preparation and thermal cycling of expanded graphite/adipic acid composite phase change materials. J Thermal Anal Calorim 129:1639–1645. https://doi.org/10.1007/s10973-017-6385-2
Yaremko AM, Silvi B, Zelsmann HR (2000) The low frequency vibrations of hydrogen bonded adipic acid crystals. J Mol Struct 520:125–130. https://doi.org/10.1016/S0022-2860(99)00327-0
Ratajczak H, Yaremko AM (1995) Theory of profiles of hydrogen stretching infrared bands of hydrogen bonded solids: models of strong coupling between the high frequency hydrogen stretching vibration and low frequency phonons. Chem Phys Lett 243:348–353. https://doi.org/10.1016/0009-2614(95)00864-Z
Tong Q, Lin HY, Qin XY, Yang RS, Guo YF, Wei XY (2020) Scenario analysis on abating industrial process greenhouse gas emissions from adipic acid production in China. Pet Sci 17:1171–1179. https://doi.org/10.1007/s12182-020-00450-0
Gopalan RS, Kumaradhas P, Kulkarni GU, Rao CNR (2000) An experimental charge density study of aliphatic dicarboxylic acids. J Mol Struct 521:97–106. https://doi.org/10.1016/S0022-2860(99)00293-8
Rahman A, Mupa M, Mahamadi C (2016) A mini review on new emerging trends for the synthesis of adipic acid from metal-nano heterogeneous catalysts. Catal Lett 146:788–799. https://doi.org/10.1007/s10562-015-1682-5
Sun J, Raza M, Sun X, Yuan Q (2018) Biosynthesis of adipic acid via microaerobic hydrogenation of cis,cis-muconic acid by oxygen-sensitive enoate reductase. J Biotechnol 280:49–54. https://doi.org/10.1016/j.jbiotec.2018.06.304
Ju JH, Oh BR, Heo SY, Lee YU, Shon JH, Kim SH, Kim YM, Seo JW, Hong WK (2020) Production of adipic acid by short and long-chain fatty acid acyl-CoA oxidase engineered in yeast Candida tropicalis. Bioprocess Biosyst Eng 43:33–43. https://doi.org/10.1007/s00449-019-02202-w
Jeeva S, Muthu S, Thomas R, Raajaraman BR, Mani G, Vinitha G (2020) Co-crystals of urea and hexanedioic acid with third order nonlinear properties: an experimental and theoretical enquiry. J Mol Struct 1202:127237. https://doi.org/10.1016/j.molstruc.2019.127237
Shanti A, Krishnan C, Selvarajan P (2014) Growth and characterization of a single crystal of urea adipic acid (UAA) – a third order nonlinear optical material. Spectrochim Acta 122:521–528. https://doi.org/10.1016/j.saa.2013.11.067
Kariem M, Kumar M, Yawer M, Sheikh HN (2017) Solvothermal synthesis and structure of coordination polymers of Nd(III) and Dy(III) with rigid isophthalic acid derivatives and flexible adipic acid. J Mol Struct 1150:438–446. https://doi.org/10.1016/j.molstruc.2017.08.111
Karlsson E, Mapelli V, Olsson L (2017) Adipic acid tolerance screening for potential adipic acid production hosts. Microb Cell Factories 16:20. https://doi.org/10.1186/s12934-017-0636-6
Ilkeda M (2003) Amino acid production processes. Adv Biochem Eng Biotechnol 79:1–35. https://doi.org/10.1007/3-540-45989-8_1
Das B, Baruah JB (2011) Assemblies of cytosine within H-bonded network of adipic acid and citric acid. J Mol Struct 1001:134–138. https://doi.org/10.1016/j.molstruc.2011.06.029
Shan N, Jones W (2003) Identification of supramolecular templates: design of solid state photoreactivity using structural similarity. Tetrahedron Lett 44:3687–3689. https://doi.org/10.1016/S0040-4039(03)00685-3
Wu HS, Cui XY, Qin XF, Strout D, Jiao H (2006) Boron nitride cages from B12N12 to B36N36: square hexagon alternants vs boron nitride tubes. J Mol Model 12:537–542. https://doi.org/10.1007/s00894-005-0042-6
Xia QY, Lin QF, Zhao W (2012) Theoretical study on the structural, vibrational and thermodynamic properties of the (Br2GaN3)n (n=1-4) clusters. J Mol Model 18:905–911. https://doi.org/10.1007/s00894-011-1126-0
Yin B, Wang G, Sa N, Huang Y (2008) Bonding analysis and stability on alternant B16N16 cage and its dimmers. J Mol Model 14:789–795. https://doi.org/10.1007/s00894-008-0303-2
Kandalam AK, Blanco MA, Pandey R (2002) Theoretical study of AlnNn, GanNn, and InnNn (n=4,5,6) clusters. J Phys Chem B 106:1945–1953. https://doi.org/10.1021/jp0140062
Costales A, Kandalam AK, Franco R, Pandey R (2002) Theoretical study of structural and vibrational properties of (AlP)n, (AlAs)n, (GaP)n, (GaAs)n, (InP)n and (InAs)n clusters with n=1,2,3. J Phys Chem B 106:1940–1944. https://doi.org/10.1021/jp013906f
Yong Y, Liu K, Song B, He P, Wang P, Li H (2012) Coalescence of BnNn fullerene: a new pathway to produce boron nitride nanotubes with small diameter. Phys Lett A 376:1465–1467. https://doi.org/10.1016/j.physleta.2012.03.011
Tsipas P, Kassavetis S, Tsoutsou D, Xenogiannopulou E, Golias E, Giamini SA, Grazianetti C, Chiappe D, Molle A, Fanciulli M, Dimoulas A (2013) Evidence for graphite like hexagonal AlN nanosheets epitaxially grown on single crystal Ag(111). Appl Phys Lett 103:251605. https://doi.org/10.1063/1.4851239
de Almeida Junior EF, de BritoMota F, de Castilho CMC, Kakanakova-Georgieva A, Gueroguiev GK (2012) Defects in hexagonal-AlN sheets by first principle calculations. Eur Phys J B 85:48. https://doi.org/10.1140/epjb/e2011-20538-6
Freitas PRQ, Gueorguiev GK, de BritoMota F, de Castilho CMC, Stafstrom S, Kakanakova-Georgieva A (2013) Reactivity of adducts relevant to the deposition of hexagonal BN from first principles calculations. Chem Phys Lett 583:119–124. https://doi.org/10.1016/j.cplett.2013.07.077
Strout DL (2000) Structure and stability of boron nitrides: isomers of B12N12. J Phys Chem A 104:3364–3366. https://doi.org/10.1021/jp994129a
Wang R, Zhang D, Liu C (2005) Theoretical prediction of a novel inorganic fullerene like family of silicon – carbon materials. Chem Phys Lett 411:333–338. https://doi.org/10.1016/j.cplett.2005.06.055
Wu H, Fan X, Kuo JL (2012) Metal free hydrogenation reaction on carbon doped boron nitride fullerene: a DFT study on the kinetic issue. Int J Hydrog Energy 37:14336–14342. https://doi.org/10.1016/j.ijhydene.2012.07.081
Beheshtian J, AhmadiPeyghan A, Bagheri Z (2012) Quantum chemical study of fluorinated AlN nanocage. Appl Surf Sci 259:631–636. https://doi.org/10.1016/j.apsusc.2012.07.088
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2012) A comparative study on the B12N12, Al12N12, B12P12 and Al12P12 fullerene like cages. J Mol Model 18:2653–2658. https://doi.org/10.1007/s00894-011-1286-y
Shokuhi Rad A, Ayub A (2016) A comparative density functional theory study of guanine chemisorptions on Al12N12, Al12P12, B12N12 and B12P12 nanocages. J Alloys Compd 672:161–169. https://doi.org/10.1016/j.jallcom.2016.02.139
Shokuhi Rad A, Ayub K (2016) Ni adsorption on Al12P12 nanocage: DFT study. J Alloys Compd 678:317–324. https://doi.org/10.1016/j.jallcom.2016.03.175
Shokuhi Rad A, Ayub K (2016) Detailed surface study of adsorbed nickel on Al12N12 nanocage. Thin Solid Films 612:179–185. https://doi.org/10.1016/j.tsf.2016.05.055
Beheshtian J, Bagheri Z, Kamfiroozi M, Ahmadi A (2011) Toxic CO detection by B12 N12 nanocluster. Microelectron J 42:1400–1403. https://doi.org/10.1016/j.mejo.2011.10.010
Soltani A, Baei MT, Taghartapeh MR, Lemeski ET, Shojaee S (2015) Phenol interaction with different nanocages with and without an electric field: a DFT study. Struct Chem 26:685–693. https://doi.org/10.1007/s11224-014-0504-5
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian, Inc., Wallingford CT
Shokuhi Rad A, Foukolaei VP (2015) Density functional study of Al-doped graphene nanostructure towards adsorption of CO, CO2 and H2O. Synth Met 210:171–178. https://doi.org/10.1016/j.synthmet.2015.09.026
Shokuhi Rad A (2016) Al-doped graphene as a new nanostructure adsorbent for some halomethane compounds : DFT calculations. Surf Sci 645:6–12. https://doi.org/10.1016/j.susc.2015.10.036
Shokuhi Rad A, Modanloujouibary Y, Foukolaei VP, Binaeian E (2016) Study on the structure and electronic property of adsorbed guanine on aluminum doped graphene: first principles calculations. Curr Appl Phys 16:527–533. https://doi.org/10.1016/j.cap.2016.02.004
Al-Otaibi JA (2020) Detailed quantum mechanical studies on bioactive benzodiazepine derivatives and their adsorption over graphene sheets. Spectrochim Acta 235:118333. https://doi.org/10.1016/j.saa.2020.118333
Kumar VSC, Panicker CY, Fun HK, Mary YS, Harikumar B, Chandraju S, Quah CK, Ooi CW (2014) FT-IR, molecular structure, first order hyperpolarizability, HOMO and LUMO analysis, MEP and NBO analysis of 2-(4-chlorophenyl)-2-oxoethyl 3-nitrobenzoate. Spectrochim Acta 126:208–219. https://doi.org/10.1016/j.saa.2014.01.145
Mary YS, Panicker CY, Sapnakumari M, Narayana B, Sarojini BK, Al-Saadi AA, Van Alsenoy C, War JA (2015) FT-IR, NBO, HOMO-LUMO, MEP analysis and molecular docking study of 1-[3-(4-fluorophenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1yl]ethanone. Spectrochim Acta 136:483–493. https://doi.org/10.1016/j.saa.2014.09.061
Parr RG, Yang WT (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc 106:4049–4050. https://doi.org/10.1021/ja00326a036
Ayers PW, Yang WT, Bartolotti LJ (2009) Fukui function. In: Chattaraj PK (ed) Chemical reactivity theory: a density functional view. CRC Press, Boca Raton, pp 255–267
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873. https://doi.org/10.1021/cr990029p
Ayers PW, Anderson JSM, Bartolotti LJ (2005) Perturbative perspectives on the chemical reaction prediction problem. Int J Quantum Chem 101:520–534. https://doi.org/10.1002/qua.20307
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Ayers PW (2007) The physical basis of the hard/soft acid/base principle. Faraday Discuss 135:161–190. https://doi.org/10.1039/b606877d
Pearson RG (1987) Recent advances in the concept of hard and soft acids and bases. J Chem Educ 64:561–567. https://doi.org/10.1021/ed064p561
Parr RG, Chattaraj PK (1991) Principle of maximum hardness. J Am Chem Soc 113:1854–1855 ja00005a072
Ayers PW, Parr RG (2000) Variational principles for describing chemical reactions: the Fukui function and chemical hardness revisited. J Am Chem Soc 122:2010–2018. https://doi.org/10.1021/ja9924039
Mary YS, Miniyar PB, Mary YS, Resmi KS, Panicker CY, Armakovic S, Armakovic SJ, Thomas R, Sureshkumar B (2018) Synthesis and spectroscopic study of three new oxadiazole derivatives with detailed computational evaluation of their reactivity and pharmaceutical potential. J Mol Struct 1173:469–480. https://doi.org/10.1016/j.molstruc.2018.07.026
Hossain H, Thomas R, Mary YS, Resmi KS, Armakovic S, Armakovic SJ, Nanda AK, Vijayakumar G, Van Alsenoy C (2018) Understanding reactivity of two newly synthetized imidazole derivatives by spectroscopic characterization and computational study. J Mol Struct 1158:176–196. https://doi.org/10.1016/j.molstruc.2018.01.029
Mary YS, Varghese HT, Panicker CY, Girisha M, Sagar BK, Yathirajan HS, Al-Saadi AA, Van Alsenoy C (2015) Vibrational spectra, HOMO, LUMO, NBO, MEP analysis and molecular docking study of 2,2-diphenyl-4-(piperidin-1yl)butanamide. Spectrochim Acta 150:543–556. https://doi.org/10.1016/j.saa.2015.05.090
Kaur M, Mary YS, Varghese HT, Panicker CY, Yathirajan HS, Siddegowda MS, Van Alsenoy C (2012) Vibrational spectroscopic, molecular structure, first hyperpolarizability and NBO studies of 4′-methylbiphenyl-2-carbonitrile. Spectrochim Acta 98:91–99. https://doi.org/10.1016/j.saa.2012.08.061
Maria, Iqbal J, Ayub K (2016) Theoretical study on nonlinear optical properties of alkali metal (Li, Na, K) doped aluminum nitride nano-cages. RSC Adv 6:94228–94235. https://doi.org/10.1039/C6RA21797D
Toosi AR, Shamouei HR, Hesari AM (2016) Influence of alkali metal superoxides on structure, electronic, and optical properties of Be12O12 nanocage: density functional theory study. Chin Phys B 25:094220. https://doi.org/10.1088/1674-1056/25/094220
Al-Otaibi JS, Almuqrin AH, Mary YS, Mary YS (2020) Comprehensive quantum mechanical studies on three bioactive anastrozole based triazole analogues and their SERS active graphene complex. J Mol Struct 1217:128388. https://doi.org/10.1016/j.molstruc.2020.128388
Almuqrin AH, Al-Otaibi JS, Mary YS, Mary YS, Thomas R (2020) Structural study of letrozole and metronidazole and formation of self-assembly with graphene and fullerene with the enhancement of physical, chemical and biological activities. J Biomol Struct Dyn. https://doi.org/10.1080/07391102.2020.1790420
Al-Otaibi JS, Mary YS, Mary YS, Kaya S, Erkan S (2020) Spectral analysis and DFT investigation of some benzopyran analogues and their self-assemblies with grapheme. J Mol Liq 317:113924. https://doi.org/10.1016/j.molliq.2020.113924
Lagunin A, Stepanchikova A, Filimonov D, Poroikov V (2000) PASS: prediction of activity spectra for biologically active substances. Bioinformatics 16:747–748. https://doi.org/10.1093/bioinformatics/16.8.747
Duhovny D, Nussinov R, Wolfson HJ (2000) Efficient unbound docking of rigid molecules. In: Gusfield et al (eds) Proceedings of the second workshop on algorithms in bioinformatics (WABI) Rome, Italy, Lecture notes in computer science, vo. 2452. Springer Verlag, pp 185–200
Schneidman-Duhovny D, Inbar Y, Nussinov R, Wolfson HJ (2005) Patchdock and Symmdock: servers for rigid and symmetric docking. Nucleic Acids Res 33:W363–W367. https://doi.org/10.1093/nar/gki481
Mary YS, Mary YS, Resmi KS, Kumar VS, Thomas R, Sureshkumar B (2019) Detailed quantum mechanical, molecular docking, QSAR prediction, photovoltaic light harvesting efficiency analysis of benzil and its halogenated analogues. Heliyon 5:e2825. https://doi.org/10.1016/j.heliyon.2019.e02825
Mary YS, Mary YS, Resmi KS, Thomas R (2019) DFT and molecular docking investigations of oxicam derivatives. Heliyon 5:e02175. https://doi.org/10.1016/j.heliyon.2019.e02175
Funding
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Author information
Authors and Affiliations
Contributions
All authors, Jamelah S.Al-Otaibi, Y.Sheena Mary, Y.Shyma Mary, and Goncagül Serdaroglu, contributed to the study conception and design, material preparation, and data collections and analysis.
Corresponding author
Ethics declarations
Ethics approval
The manuscript is prepared in compliance with the Ethics in Publishing Policy as described in the Guide for Authors.
Consent to participate
The manuscript is approved by all authors for publication.
Consent for publication
The consent for publication was obtained from all participants.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Otaibi, J.S., Mary, Y.S., Mary, Y.S. et al. Adsorption of adipic acid in Al/B-N/P nanocages: DFT investigations. J Mol Model 27, 113 (2021). https://doi.org/10.1007/s00894-021-04742-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04742-z