Abstract
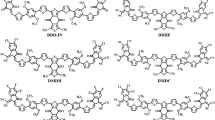
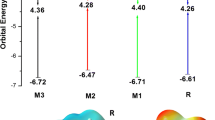
A recently synthesized photoactive donor named fluorinated thienyl–substituted benzodithiophene (DRTB-FT), modified with four novel end capped acceptor molecules, has been investigated through different electrical, quantum, and spectrochemical techniques for its enhanced electro-optical and photovoltaic properties. DRTB-FT was connected to 2-methylenemalononitrile (D-1), 2-methylene-3-oxobutanenitrile (D-2), 2-(2-methylene-3-oxo-2,3-dihydro-1H-inden-1-ylidene) malononitrile (D-3), and 3-methyl-5methylene-2-thioxothiazolidin-4-one (D-4) as terminal acceptor moieties. The architectural D-1 and D-3 molecules owe reduced optical band gap of 2.45 and 2.28 eV benefited from A-D-A configuration and have broaden maximum absorption band (λmax) at 617 and 602 nm in polar organic solvent (chloroform). Reduced optical band gap sets the ease for enhanced absorption. Reorganization energy of electron (λe) of D-3 molecule (0.00397 eV) was smaller among all which disclosed its greater mobility of conducting electrons (ICT). Larger values of dipole moment (μ) of D-1 (5.939 Debye) and D-3 (3.661 Debye) molecules in comparison to R indicated greater solubilities of the targeted molecules. Among the tailored molecules, D-3 showed the lowest binding energy of 0.25 eV in solvent phase and 0.08 eV in gaseous phase. The voltaic strength of the designed molecules was examined with respect to fullerene derivative (PC61BM) which exposed that D-1 is the best choice for achieving higher PCE. TDM (transition density matrix), DOS (density of states) analysis, and binding energies all were estimated at MPW1PW91/6-31G (d, p) level of DFT (density functional theory).
Graphical abstract

All the architecture molecules show reduced band gap and high electron transfer rate due to the lowest reorganization energy (RE) of electron. The results show that there is greater contribution of acceptor and conjugated donor core towards the total absorption into the visible region of the spectrum. When tailored molecules D-1, D-2, D-3, and D-4 were blended with fullerene derivative polymer (PC61BM), they give high values of voltage at zero current level (Voc) compared to R.








Similar content being viewed by others
Data Availability
All data will be available if required.
Code availability
Not applicable
References
Schlör H, Fischer W, Hake J-F (2012) The meaning of energy systems for the genesis of the concept of sustainable development. Appl Energy 97:192–200
Güney T (2019) Renewable energy, non-renewable energy and sustainable development. International Journal of Sustainable Development & World Ecology 26(5):389–397
Menyah K, Wolde-Rufael Y (2010) CO2 emissions, nuclear energy, renewable energy and economic growth in the US. Energy Policy 38(6):2911–2915
Boyle, G.. (2004) Energy for a sustainable future: renewable energy: Open University Press.
Panwar N, Kaushik S, Kothari S (2011) Role of renewable energy sources in environmental protection: a review. Renew Sust Energ Rev 15(3):1513–1524
Krebs FC et al (2014) 25th anniversary article: rise to power–OPV-based solar parks. Adv Mater 26(1):29–39
Miles RW, Zoppi G, Forbes I (2007) Inorganic photovoltaic cells. Mater Today 10(11):20–27
Farhat A et al (2020) Tuning the optoelectronic properties of subphthalocyanine (SubPc) derivatives for photovoltaic applications. Opt Mater 107:110154
Yao H et al (2016) Molecular design of benzodithiophene-based organic photovoltaic materials. Chem Rev 116(12):7397–7457
Mishra A, Bäuerle P (2012) Small molecule organic semiconductors on the move: promises for future solar energy technology. Angew Chem Int Ed 51(9):2020–2067
Scharber MC, Sariciftci NS (2013) Efficiency of bulk-heterojunction organic solar cells. Prog Polym Sci 38(12):1929–1940
Liu M et al (2020) Naphthalene-diimide-based ionenes as universal interlayers for efficient organic solar cells. Angew Chem Int Ed 59(41):18131–18135
Yao J et al (2020) Cathode engineering with perylene-diimide interlayer enabling over 17% efficiency single-junction organic solar cells. Nat Commun 11(1):1–10
Firdaus Y et al (2019) Key parameters requirements for non-fullerene-based organic solar cells with power conversion efficiency> 20%. Advanced Science 6(9):1802028
Cui Y et al (2019) Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat Commun 10(1):1–8
Zhan C, Zhang X, Yao J (2015) New advances in non-fullerene acceptor based organic solar cells. RSC Adv 5(113):93002–93026
Huo Y, Zhang H-L, Zhan X (2019) Nonfullerene all-small-molecule organic solar cells. ACS Energy Letters 4(6):1241–1250
Liu C et al (2020) All-small-molecule organic solar cells based on a fluorinated small molecule donor with high open-circuit voltage of 1.07 V. Frontiers in Chemistry 8:329
Bicout, D. and M. Field (1996) Quantum mechanical simulation methods for studying biological systems.
Dennington R, Keith TA, Millam JM (2008) GaussView 5. Gaussian. Inc., Wallingford
Frisch, M., et al. (2009) Gaussian 09, Revision d. 01, Gaussian. Inc., Wallingford CT. 201.
Civalleri B et al (2008) B3LYP augmented with an empirical dispersion term (B3LYP-D*) as applied to molecular crystals. CrystEngComm 10(4):405–410
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393(1-3):51–57
Adamo C, Barone V (1998) Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the m PW and m PW1PW models. J Chem Phys 108(2):664–675
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10(44):6615–6620
Yu C, Hao X, Shen Q (2010) Illustration of origin 8.0. Chemical Industry Press, Beijing
Shehzad RA et al (2020) Designing of benzothiazole based non-fullerene acceptor (NFA) molecules for highly efficient organic solar cells. Computational and Theoretical Chemistry:112833
Tenderholt A (2006) PyMOlyze, Version 1.1. Stanford University. CA, Stanford
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592
Marcus RA (1993) Electron transfer reactions in chemistry. Theory and experiment. Rev Mod Phys 65(3):599
Fujisawa J-i (2014) An unusual mechanism for HOMO–LUMO gap narrowing in a minimal near-IR dye generated by the deprotonation of bis(dicyanomethylene)indan. Chem Phys Lett 608:355–359
Musawwir A et al (2021) Theoretical and computational study on electronic effect caused by electron withdrawing/electron-donating groups upon the coumarin thiourea derivatives. Computational and Theoretical Chemistry 1201:113271
Saleem R et al (2021) Designing of small molecule non-fullerene acceptors with cyanobenzene core for photovoltaic application. Computational and Theoretical Chemistry 1197:113154
Oehlenschlaeger KK et al (2014) Adaptable hetero Diels-Alder networks for fast self-healing under mild conditions. Adv Mater 26(21):3561–3566
Yang L et al (2019) Theoretical studies on 4H-cyclopenta [2, 1-b: 3, 4-b′] dithiophene-based Windmill-shaped nanogrids with low reorganization energies. Chem Phys 516:191–198
Collado-Fregoso E et al (2015) Increased exciton dipole moment translates into charge-transfer excitons in thiophene-fluorinated low-bandgap polymers for organic photovoltaic applications. Chem Mater 27(23):7934–7944
Farhat A et al (2021) Designing and theoretical characterization of benzodithiophene dione based donor molecules for small molecule organic solar cells. Optik:167098
Ans M et al (2019) Designing indacenodithiophene based non-fullerene acceptors with a donor–acceptor combined bridge for organic solar cells. RSC Adv 9(7):3605–3617
Etienne T (2015) Transition matrices and orbitals from reduced density matrix theory. J Chem Phys 142(24):244103
Li Y, Ullrich C (2011) Time-dependent transition density matrix. Chem Phys 391(1):157–163
Mehboob MY et al (2020) Designing of benzodithiophene core-based small molecular acceptors for efficient non-fullerene organic solar cells. Spectrochim Acta A Mol Biomol Spectrosc:118873
Azzouzi M, Kirchartz T, Nelson J (2019) Factors controlling open-circuit voltage losses in organic solar cells. Trends in Chemistry 1(1):49–62
Dennler G et al (2008) Design rules for donors in bulk-heterojunction tandem solar cells towards 15% energy-conversion efficiency. Adv Mater 20(3):579–583
Acknowledgements
The authors acknowledge the financial and technical support from Punjab Bio-energy Institute (PBI), University of Agriculture Faisalabad (UAF), Pakistan.
Funding
Funding acquisition from Punjab Bio-energy Institute (PBI), University of Agriculture Faisalabad (UAF), Pakistan.
Author information
Authors and Affiliations
Contributions
Usama Mubashar: writing original draft; Afifa Farhat: formal analysis; Rasheed Ahmad Khera: data curation; Rasheed Ahmad Khera: funding acquisition; Naseem Iqbal: formal analysis, data curation; Rabi Saleem: data curation; Javed Iqbal: funding acquisition, formal analysis, data curation.
Corresponding authors
Ethics declarations
Ethics approval
We approved all ethics.
Consent to participate
Not applicable
Consent for publications
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 68 kb)
Supporting information (SI-1) include Cartesian coordinates of internally optimized geometries of all molecules (reference R, and architecture molecules D-1, D-2, D-3, and D-4 along X-, Y-, and Z-axis at MPW1PW91/6-31G (d, p) level of density functional theory (DFT).
Rights and permissions
About this article
Cite this article
Mubashar, U., Farhat, A., Khera, R.A. et al. Designing and theoretical study of fluorinated small molecule donor materials for organic solar cells. J Mol Model 27, 216 (2021). https://doi.org/10.1007/s00894-021-04831-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04831-z