Abstract
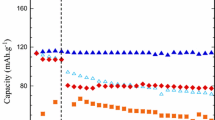
A study of the electrochemical behavior of LiMnPO4 prepared by RAPET method in different aqueous electrolytes using cyclic voltammetry (CV), galvanostatic charge–discharge experiments, and electrochemical impedance spectroscopy is reported. CV peak current is proportional to the square root of scan rate under 0.2 mV s−1. The system satisfied the required conditions for a reversible system with a resistive behavior. LiMnPO4 was found to undergo proton insertion at lower concentrations of electrolyte. At higher concentrations or saturated solutions of electrolytes, lithium insertion/de-insertion becomes the main reaction though the effect of proton insertion/de-insertion reaction cannot be ignored. Electrochemical insertion/de-insertion of lithium in LiMnPO4 was studied using EIS technique. The kinetic parameter, charge transfer resistance (R ct), obtained by simulating the experimental impedance data with an equivalent circuit showed a minimum at the potential close to the CV peak potential. The cell LiTi2(PO4)3/5 M LiNO3/LiMnPO4 delivers a discharge capacity of 84 mAh g−1 in the first cycle at an applied current of 0.2 mA cm−2 and it retains its initial capacity over 50 cycles with good rate capability.














Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) J Electrochem Soc 144:1188–1194
Manjunatha H, Suresh GS, Venkatesha TV (2011) J Solid State Electrochem 15:431–445
Yamada A, Hosoya M, Chung S-C, Hinokuma K, Kudo Y, Liu K-Y (2002) In: Manthiram A, Kumta PN, Sundaram SK, Ceder G (eds) Materials for electrochemical energy conversion and storage. American Ceramic Society, Westerville, pp 189–204
Yamada A, Hosoya M, Chung S-C, Hinokuma K, Kudo Y, Liu K-Y (2001) In: Nadri G, Koetz R, Dcrosatti B, Moro PA, Takeuchi ES (eds) Advanced batteries and supercapacitors. The Electrochemical Society, Pennington
MacNeil DD, Lu Z, Chen Z, Dahn JR (2002) J Power Sources 108:8–114
Yamada A, Chung S-C, Hinokuma K (2001) J Electrochem Soc 148:A224–A229
Delacourt C, Poizot P, Morcrette M, Tarascan JM, Masquelier C (2004) Chem Mater 16:93–99
Gummov RJ, Thackery MM (1993) J Electrochem Soc 140:3365–3368
Guohua L, Ikuta H, Uchida T, Wakihara M (1996) J Electrochem Soc 143:178–182
Li G, Azuma H, Tohda M (2002) Electrochem Solid-State Lett 5:A135–A137
Yamada A, Chung S-C (2001) J Electrochem Soc 148:A960–A967
Bramnik NN, Ehrenberg H (2008) J Alloys Compd 464:259–264
Bakenov Z, Taniguchi I (2010) Electrochem Commun 12:75–78
Huang H, Yin S-C, Nazar LF (2001) Electrochem Solid-State Lett 4:A170–A172
Minakshi M, Singh P, Thurgate S, Prince K (2006) Electrochem Solid-State Lett 9:A471–A474
Minakshi M, Pandey A, Blackford M, Ionescu M (2010) Energy Fuel 24:6193–6197
Pol VG, Pol SV, Gedanken A, Kessler VG, Seisenbaeva GA, Sung M, Asai S (2005) J Phys Chem B 109:6121–6125
Pol SV, Pol VG, Kessler VG, Seisenbaeva GA, Sung M, Asai S, Gedanken A (2004) J Phys Chem B 108:6322–6327
Pol SV, Pol VG, Kessler VG, Seisenbaeva GA, Sung M, Asai S, Gedanken A (2004) Chem Mater 16:1793–1798
Pol VG, Pol SV, Gedanken A (2008) J Phys Chem C 112:6627–6637
Chen G, Richardson TJ (2009) J Electrochem Soc 156:A756–A762
Delacourt C, Poizot P, Morcrette M, Tarascon JM, Masquelier C (2004) Chem Mater 16:93–99
Yu DYW, Fietzek C, Weydanz W, Donoue K, Inoue T, Kurokawa H, Fujitani S (2007) J Electrochem Soc 154:A253–A257
Molenda J, Ojczyk W, Swierczek K, Zajac W, Liu R-S Krok F Dygas J (2006) Solid State Ionics 177:2617–2624
Lee J-W, Pyun S-II (2004) Electrochim Acta 49:753–761
Manjunatha H, Mahesh KC, Suresh GS, Venkatesha TV (2011) Electrochim Acta 56:1439–1446
Shivashankaraiah RB, Manjunatha H, Mahesh KC, Suresh GS, Venkatesha TV (2011) J Solid State. Electrochem. doi:10.1007/s10008-011-1520-7
Manjunatha H, Venkatesha TV, Suresh GS (2011) Electrochim Acta 58:247–257
Sinha NN, Ragupathy P, Vasan HN, Munichandraiah N (2008) Int J Electrochem Soc 3:691–710
Ho C, Raistrick ID, Huggins RA (1980) J Electrochem Soc 127:343–350
Wang GJ, Qu QT, Wang B, Shi Y, Tian S, Wu YP, Holze R (2009) J Power Sources 189:503–506
Wang GJ, Qu QT, Wang B, Shi Y, Tian S, Wu YP, Holze R (2009) Electrochim Acta 54:1199–1203
Acknowledgements
The authors gratefully acknowledge the financial support from the Department of Science and Technology, Government of India. They wish to thank Sri. A. V. S. Murthy, honorary secretary, Rashtreeya Sikshana Samiti Trust, Bangalore, and Dr. P. Yashoda, Principal, S.S.M.R.V. Degree College, Bangalore, for their support and encouragement. They thank the Department of Chemistry, St. Joseph’s College, for XRD data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjunatha, H., Venkatesha, T.V. & Suresh, G.S. Electrochemical studies of LiMnPO4 as aqueous rechargeable lithium–ion battery electrode. J Solid State Electrochem 16, 1941–1952 (2012). https://doi.org/10.1007/s10008-011-1593-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-011-1593-3