Abstract
Methane (CH4) emissions from Arctic tundra are an important feedback to global climate. Currently, modelling and predicting CH4 fluxes at broader scales are limited by the challenge of upscaling plot-scale measurements in spatially heterogeneous landscapes, and by uncertainties regarding key controls of CH4 emissions. In this study, CH4 and CO2 fluxes were measured together with a range of environmental variables and detailed vegetation analysis at four sites spanning 300 km latitude from Barrow to Ivotuk (Alaska). We used multiple regression modelling to identify drivers of CH4 flux, and to examine relationships between gross primary productivity (GPP), dissolved organic carbon (DOC) and CH4 fluxes. We found that a highly simplified vegetation classification consisting of just three vegetation types (wet sedge, tussock sedge and other) explained 54% of the variation in CH4 fluxes across the entire transect, performing almost as well as a more complex model including water table, sedge height and soil moisture (explaining 58% of the variation in CH4 fluxes). Substantial CH4 emissions were recorded from tussock sedges in locations even when the water table was lower than 40 cm below the surface, demonstrating the importance of plant-mediated transport. We also found no relationship between instantaneous GPP and CH4 fluxes, suggesting that models should be cautious in assuming a direct relationship between primary production and CH4 emissions. Our findings demonstrate the importance of vegetation as an integrator of processes controlling CH4 emissions in Arctic ecosystems, and provide a simplified framework for upscaling plot scale CH4 flux measurements from Arctic ecosystems.
Similar content being viewed by others
Introduction
The Arctic is warming at nearly double the global rate (IPCC 2013). A temperature increase of approximately 6°C is predicted by the end of the twenty-first century in northern high latitudes (IPCC 2013), leading to major changes in hydrological and thermal regimes, which in turn will heavily influence the direction and magnitude of the Arctic carbon (C) balance (Oechel and others 2000; Chapin and others 2005). One of the greatest concerns is the potential for increases in methane (CH4) emissions from tundra ecosystems to the atmosphere. Arctic tundra ecosystems currently account for approximately 8–30 Tg CH4 y−1 released to the atmosphere (Christensen 1993; McGuire and others 2012; Olefeldt and others 2013). For context, global emission rate from both natural and anthropogenic sources is approximately 500–600 Tg CH4 y−1 (Dlugokencky and others 2011). As CH4 has 28.5 times the global warming potential of carbon dioxide (CO2) over 100 years (IPCC 2013), increased emissions are an important positive feedback from the arctic region to global climate (Forster and others 2007; IPCC 2013), further warming the climate system, leading to permafrost degradation and therefore increased emissions.
To reduce uncertainties in predicting future climate, an improvement in modelling of CH4 emissions is urgently required (Matthews and Fung 1987; Petrescu and others 2010; Bohn and others 2015). Currently, many carbon cycle models disagree on the response of CH4 fluxes to climate change (Melton and others 2013; Bohn and others 2015). Despite many empirical studies in the Arctic, the controls on CH4 fluxes remain uncertain, most notably for large and heterogeneous tundra ecosystems. Substantial variation in CH4 flux has been observed even over distances of just a few meters (Kutzbach and others 2004; Olivas and others 2010; Kade and others 2012), making predictions of CH4 fluxes from these landscapes particularly challenging. Previous studies have identified important environmental controls on the spatial heterogeneity of CH4 fluxes as water table height, active layer thaw depth, soil moisture and soil temperature (Zona and others 2009; Sturtevant and others 2012; Zona and others 2016). Vegetation also plays an important role, as it provides substrate for methanogenesis and increases CH4 transport and atmospheric emissions (Whiting and Chanton 1993; King and others 2002; Bridgham and others 2013).
Because vegetation type is a product of many environmental variables that also control CH4 emissions, vegetation type itself may be a good integrator of conditions controlling CH4 flux, allowing prediction of CH4 fluxes from assessment of vegetation cover instead of measuring multiple environmental variables. However, many studies to date which have examined the vegetation and environmental controls on CH4 fluxes in northern ecosystems are focused on single sites or vegetation types (Christensen and others 2003, 2004; Zona and others 2009; Olivas and others 2010). Thus, further assessment of the consistency of relationships between vegetation, environmental controls and CH4 fluxes across multiple tundra ecosystem types is still lacking (Fox and others 2008; Sachs and others 2010).
In particular, improved understanding of relationships between gross primary productivity (GPP), above-ground plant community cover and CH4 fluxes is important for modelling future CH4 emissions. Plants provide the substrate for methanogenesis through transfer of labile C to the rhizosphere, and thus many climate models assume an increase in plant productivity in arctic tundra wetlands will result in higher CH4 emissions (Melton and others 2013). However, GPP and CH4 fluxes are very rarely measured at the same time across a variety of tundra ecosystems, meaning the validity of this assumption has yet to be tested across multiple tundra sites. In addition to providing substrate, plants such as sedges enable the transport of CH4 from anoxic zones of production (methanogenesis) to the atmosphere, bypassing oxic zones where CH4 consumption (methanotrophy) may occur (Torn and Chapin 1993; Bubier 1995; Bubier and others 1995; King and others 1998; Ström and others 2003; McEwing and others 2015). This capacity to facilitate transport can differ widely between plant species or growth forms (Koelbener and others 2010; Dorodnikov and others 2011), and the relative importance of substrate limitation and plant transport are not well established (Schimel 1995; Joabsson and Christensen 2001; King and others 2002). A strong relationship between CH4 fluxes and DOC may indicate supply limitation rather than transport-limitation of ecosystem CH4 fluxes (Neff and Hooper 2002), and thus inform the conceptual approach to modelling these ecosystems.
To address these issues, we measured CH4 fluxes in contrasting micro-topographic positions in multiple Arctic vegetation types in four sites spanning a 300 km latitudinal gradient in northern Alaska, and investigated the vegetation and environmental controls of these fluxes.
This study provides critical advances on the work undertaken by McEwing and others (2015) using the same field sites. Our investigations present a more extensive analysis of the vegetation communities present, a wider range of environmental and vegetation variables, and a much larger sample size than the McEwing and others 2015 study.
We hypothesised that (i) the dominant overall control on CH4 fluxes is water table depth across different vegetation types, (ii) when the water table is below the surface, the most important controls are the presence of sedges, and (iii) vegetation type will be a good predictor of CH4 fluxes across arctic tundra, despite variation in environmental variables within the growing season.
Materials and Methods
Site Description
The study was performed at four sites in northern Alaska: two in Barrow (Barrow-BEO 71° 16′ 52′′N, 156° 36′ 44′′W and Barrow-BES 71° 16′ 51.61′′N, 156° 36′ 44.44′′W), one in Atqasuk (70° 28′ 40′′N, 157° 25′05′′W) and one in Ivotuk (68.49°N, 155.74°W) (Figure 1).
Barrow is located within the Arctic Coastal Plain, where the landscape consists of thaw lake basins and areas of interstitial tundra, with approximately 65% of ground covered by low-, high- and flat-centred ice-wedge polygons (Brown 1967; Billings and Peterson 1980). The Barrow-BES site (a drained thaw lake basin) has modest development of low centre polygons, and usually has a water table above the surface of the soil due to its low elevation (Zona and others 2009). The Barrow-BEO site (500 m west of Barrow-BES) is substantially drier, with well-developed high-centre, flat-centre and low-centre polygons. Vegetation is predominantly wet graminoid-moss communities (Raynolds and others 2005). Soils within the Barrow field sites are classified as Gelisols with three suborders (Turbels, 77%, Orthels, 8.7% and organic soils, 1% underlain by permafrost) (Bockheim and others 1999, 2001) within 100 cm of the surface, with a soil organic matter (SOM) depth of between 0 and more than 30 cm.
Atqasuk is located approximately 100 km south of Barrow with well-developed, low-centred, ice-wedge polygons with well-drained rims (Komarkova and Webber 1980; Oechel and others 2014). The vegetation consists mainly of wet sedge-moss communities and, unlike Barrow, has moist shrub and tussock sedge communities on higher microsites (Raynolds and others 2005). Soils are approximately 95% sand and 5% clay and silt to a depth of 1 m (Walker and others 1989), with a SOM layer depth between 0 and 19 cm, silt loam-textured mineral material and underlying permafrost (Michaelson and Ping 2003; Kwon and others 2006).
Ivotuk is the southernmost site, 300 km south of Barrow. It lacks substantial ice-wedge polygon development and comprises a gentle north-west facing slope and a lower lying wet meadow on the margins of a stream. Vegetation is predominantly tussock sedge, dwarf shrub, moss communities (Raynolds and others 2005), and the soils are classified as mostly Ruptic Pergelic and Cryaquept acid (Edwards and others 2000) with a SOM layer depth of between 4 and more than 30 cm [SOM content of between 25 and 50% C (Michaelson and Ping 2003)].
Vegetation Types
The four sites are located in major vegetation types, including graminoid-dominated wetlands, tussock graminoid tundra on sandy substrates and tussock graminoid tundra on non-sandy substrates (Raynolds and others 2006) (Figure 1). Within each of these broad vegetation types, substantial variability of vegetation communities was identified (Table 1; Figure 2). To enable installation of flux collars early in the growing season, the main vegetation types present were identified by walkover surveys at each site, using aerial images (WorldView2, DigitalGlobe, USA) to identify potential landscape units for further investigation. Vegetation maps and descriptions for all sites (Webber 1978; Komarkova and Webber 1980; Edwards and others 2000) were also examined to maximise consistency with existing classifications.
Walkover surveys showed that the vegetation at Barrow-BEO on polygon high centres consisted of Polytrichum moss and lichen-dominated communities with few vascular plants. Polygon rims and flat centres were dominated by a mixture of graminoids, including Eriophorum russeolum (sedge), Poa arctica (grass) and Luzula arctica (rush), with Dicranum mosses, liverworts and frequent lichens. The sedge Carex aquatilis dominated sparse vascular plant canopies in polygon troughs, polygon low centres and the drained lake basin at Barrow-BES.
At Atqasuk, tussock tundra communities on dry ridges and plateaus comprised 21% Eriophorum vaginatum tussocks and 79% inter-tussock areas (determined by visually estimating proportions of tussocks in 60 randomly placed 1 m2 plots in the study area). Inter-tussock areas were dominated by the moss Aulocomnion turgidum, evergreen dwarf shrubs and the forb Rubus chamaemorus, with occasional Carex bigelowii. Permanent and ephemeral pools contained E. angustifolium and E. russeolum-dominated vegetation with no moss.
At Ivotuk, the tussock tundra on flat ground consisted of 57% E. vaginatum tussocks, 42% inter-tussock vegetation and 1% moss-dominated hollows (determined as for Atqasuk). The inter-tussock vegetation was dominated by Sphagnum moss, with less than 15% cover of a variety of evergreen and deciduous dwarf shrubs, R. chamaemorus and E. vaginatum. Moss-dominated hollows lacked vascular plants, and supported continuous cover of Sphagnum sp., Drepanocladus sp. or liverworts beneath standing water. The wet sedge meadow was dominated by tall C. aquatilis above low-growing deciduous shrubs Salix pulchra and Betula nana, and abundant Sphagnum moss (Table 1).
The vegetation within the flux collars was subsequently surveyed at peak season (Ivotuk 18th July 2014, Barrow-BEO 22nd July 2014, Barrow-BES 23rd July 2014, Atqasuk 29th July 2014). Percentage cover of all vascular and non-vascular plant species was recorded as 0.1 (present), 1 (occasional, few individuals) or 3 (occasional, more individuals), and to the nearest 5% thereafter. Vascular plant identifications were made in the field according to Hultén (1968), and non-vascular plant identifications according to Vitt and others (1988). Nomenclature follows PLANTS database (USDA 2014).
CH4 and CO2 Measurements
At each site, PVC collars (height 15 cm × diameter 20 cm) were placed in all micro-topographic positions (Table 1; Figure 2). Collars were inserted upon thaw (late June) in 2014 using a serrated knife. A total of six replicate collars (Barrow-BEO) or seven (Atqasuk and Ivotuk) were placed in each vegetation type to a depth of approximately 15 cm, totalling 12 collars in Barrow-BES, 30 in Barrow-BEO, 21 in Atqasuk and 28 in Ivotuk (Table 1).
CH4 and CO2 fluxes at Barrow-BEO/Barrow-BES were measured on 29th June, 10th, 22nd July, 7th, 15th and 22nd August, at Atqasuk on 2nd, 3rd, 27th, 30th July and 11th and 13th August and at Ivotuk on 20th, 21st, 22nd June, 17th, 19th July and 20th August 2014. Due to proximity and accessibility of the Barrow sites, both were measured a total of six times, whereas the remote locations of Atqasuk and Ivotuk allowed only three visits during the summer. It was not possible to measure the “wet meadow” collars at Ivotuk during the first visit. Measurements were made at a similar time of day at all sites (10 am–3 pm).
An LGRTM, Ultraportable Greenhouse Gas Analyser (UGGA), Model 915-0011 (Los Gatos, Research, Palo Alto, CA, USA) connected to a cylindrical plexiglass chamber (H: 500 × D: 215 mm) via inlet and outlet tubing (2390 × 2 mm Bev-A-line) was used to measure CH4 and CO2 fluxes (Figure S1). The UGGA was used to measure both CH4 and CO2 concentrations using a closed system with a 1 Hz sampling rate. The chamber was left in place for two minutes to achieve a stable increase in CH4 and CO2 concentration within the chamber headspace. After measurement, the chamber was removed to re-establish ambient gas concentrations, then covered with a black felt cover and placed back on the collar for a further two minutes. Net ecosystem exchange of CO2 (net ecosystem exchange, NEE) measured with transparent chamber and ecosystem respiration (ER) was measured with opaque chamber (hood) that is used to calculate gross primary productivity (GPP = NEE + ER). Gas fluxes were calculated using the linear slope fitting technique (Pihlatie and others 2013).
Environmental and Vegetation Variables
Air temperature was measured half-hourly at 1.5 m above the surface (Vaisala HMP45C, Helsinki, Finland) at each of the sites. Soil temperature, thaw depth, pH, water table depth, soil moisture and sedge height were measured each time flux measurements were collected with the portable chamber system in each chamber collar. Soil temperature was measured at depths of 5 and 10 cm from the soil surface using an ATC temperature probe and a Handheld Data Logging Meter (HHWT-SD1 Series OMEGA Engineering, Stamford, Connecticut, USA), and volumetric soil moisture within the top 5 cm of the soil horizon using a TDR 300 (FieldScout, Spectrum technologies, Aurora, Illinois). Thaw depth was determined using a metal probe pushed into the soil, and depth recorded from the top of the moss layer as described in Zona and others (2009). Soil pH was measured at 5-cm depth from the soil surface using a PHE-1311 pH probe (HHWT-SD1 Series OMEGA Engineering, Stamford, Connecticut, USA) and a Handheld Data Logging Meter. Water table depth (relative to the ground surface) was measured in 20-mm-diameter PVC pipes which were drilled with holes every 1 cm, and inserted at the start of the growing season adjacent to each chamber collar. Sedge height was measured from the top of the moss layer to the tallest green leaf.
Percentage cover of sedges, grasses, evergreen shrubs, deciduous shrubs, forbs, mosses and lichens were recorded in the field once at peak season, and then compared with photographs taken on each flux measurement date to produce percentage cover estimates for all flux dates. Total numbers of sedge tillers per collar were counted once early in the growing season, and the number of tillers of each species was determined at peak season when sufficient material was above-ground for identification.
Dissolved Organic Carbon (DOC)
Soil pore water was collected from Barrow-BEO on 11th July 2014, Barrow-BES on 26th July 2014, Atqasuk on 10th July 2014 and Ivotuk on 18th and 19th July 2014. Additional samples were collected from Barrow-BEO on 26th July 2014. All samples were collected adjacent to chamber collars in 10-ml plastic vacutainer tubes (BD 367985) connected to 2.5-mm rhizons (Rhizosphere Research Products, Wageningen, The Netherlands). The soil was punctured and rhizons were inserted vertically to approximately 10 cm at Barrow-BEO, Barrow-BES and Ivotuk. Rhizons were inserted at an angle in Atqasuk at a depth <10 cm due to shallow active layer thaw. Vacutainers were recovered within 2–12 h and put into storage at 4°C the same day they were collected for Barrow-BEO and Barrow-BES and within 24–48 h for Atqasuk and Ivotuk, and subsequently air-freighted to San Diego State University under refrigeration. DOC was measured colorimetrically, in duplicate, on a SpectraMax 190 spectrophotometer using the methods of Bartlett and Ross (1988).
Statistical Analyses
Plant species composition data were analysed using hierarchical cluster analysis and non-metric multidimensional scaling (NMDS). Hierarchical two-way cluster analyses were carried out in PC-Ord version 6 (MjM Software, Gleneden Beach, Oregon, USA). NMDS analyses were undertaken using a Bray–Curtis distance measure, two dimensions (following examination of the stress plots of three runs), data auto-transformation (Wisconsin double and square root transformations). Following ordination, abiotic and biotic gradients and estimated peak season CH4 flux rates (obtained by taking an average of all available measurements in July and August in each vegetation type, and then averaging over the two months) were fitted as vectors to the NMDS plot. NMDS analyses were carried out in R version 3.1.0 (R Core Team 2014) using the vegan package (Oskanen and others 2013).
The significance of the vegetation (percent cover of plant functional groups (Chapin and others 1996), maximum vegetation height, number of sedge tillers) and environmental variables (soil temperature, soil moisture, water table, active layer thaw depth, DOC and pH) in explaining the rate of CH4 emissions was determined using multiple regression models. As CH4 light and dark (felt cover) flux measurements were found to be not significantly different, each light and dark flux was averaged together for further statistical analysis. CH4 fluxes were log transformed (CH4 fluxes +0.2) to meet normality and homoscedasticity assumptions required in the analyses.
Multiple regression models were run following the approach of Crawley (2012) to identify non-linear relationships and significant two-way interactions. The results were used to create an initial maximal model using the main fixed effects plus quadratic and two-way interactions. The minimum model was obtained by sequential removal of non-significant terms and models were compared using Akaike information criterion (AIC). Collar name was included as a random intercept term to account for the repeated measures taken across all sites. Model fits were plotted and examined visually for conformation with assumptions. The methods of Nakagawa and Schielzeth (2013) were used to calculate marginal \( R^{ 2} \left( {R^{ 2}_{{{\text{GLMM}}({\text{m}})}} } \right) \), which describes the proportion of the variance in the data explained by fixed effects, and conditional \( R^{ 2} \left( {R^{ 2}_{{{\text{GLMM}}({\text{c}})}} } \right) \) which describes the proportion of the variance explained by both fixed and random effects. Interactions were interpreted using the methods of Aiken and West (1991).
A further, simplified multiple regression model containing only the most significant main effects from the initial model was also fit to assess the proportion of the variation which could be explained by these factors alone. In this simplified model, the number of levels of the vegetation factor (included as a categorical variable) was reduced by repeatedly combining the most similar vegetation categories and refitting the model until no further reduction in AIC could be achieved (Figure 3). Different combinations of the different vegetation factors were used to evaluate their contribution to the overall variance explained.
Results
Vegetation Communities
Analyses of vegetation composition using hierarchical clustering and NMDS showed close groupings of flux collars (grouped based on visual inspection of the ordination diagram) according to the micro-topographic and microhabitat units in which they were originally placed (Figure 4A). A total of six “collar-scale” vegetation categories were identified from examination of the cluster analysis output and NMDS, which reflected broad, cross-site communities of dominant plant functional type (Chapin and others 1996) within the measurement collars (Figures 2, 4A). Further divisions within the analyses separated these groups by site, reflecting the regional differences in temperature, floristics and substrate patterns found across northern Alaska (Raynolds and others 2005). Vegetation composition was most strongly correlated with soil moisture variables, with a significant but weaker relationship with pH (Figure 4B; Table 1). Peak season CH4 flux was also significantly but not strongly correlated with the NMDS ordination of communities (Figure 4B; Table 1).
Non-metric multi-dimensional scaling (NMDS) ordination of flux collar vegetation communities. A Ordination based on species composition, coloured by site and topographic position and showing five collar vegetation groups (i) “moss-lichen” communities, (ii) “dry graminoid” communities, (iii) “tussock tundra” communities, (iv) “wet sedge” communities, (v) “moss-shrub” communities and (vi) “moss only”, B ordination biplot showing direction and strength of correlations of ordination with environmental variables, vegetation variables and CH4 flux
Environmental and Vegetation Variability
Air temperature ranged from between 2.1 and 13.2°C in Barrow-BEO and Barrow-BES, 4.1 and 10.9°C in Atqasuk and between 3 and 14°C in Ivotuk during the 29th June to 22nd August 2014 measurement period. Soil temperature (taken as spot measurements during each gas flux measurement) at 10-cm depth below the surface ranged from 0.3–4.3°C at the Barrow-BEO and Barrow-BES sites, 1.1–8.0°C in Atqasuk and 0.3–9.8°C in Ivotuk across the field campaign (Figure 5A). Active layer depth, reached a maximum depth of 45 and 38 cm in Barrow-BEO and Barrow-BES, respectively, 58 cm in Atqasuk and 70 cm in Ivotuk (Figure 5B). pH across all vegetation communities and all micro-topographic positions and sites was acidic—neutral (ranging between 4.2 and 5.4).
Edaphic conditions A temperature at 10-cm depth, B thaw depth, measured at four Alaskan arctic field sites during CH4 chamber flux measurements in summer 2014. Micro-topographic position in the landscape is indicated by text labels beneath x axis. Bars are mean ± standard error for each date (n = 6 or 7). na data not available
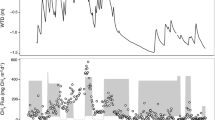
The micro-topography was tightly linked to the water table depth, with the deepest water table depths found in the high and flat centre and rims (Fig 6B). The low centres, troughs and the drained lake collars had water table depths either at or above the surface (top of moss layer) throughout the majority of the field campaign.
A CH4 fluxes and B water table depth (positive = standing water, negative = water table below the soil surface) measured at four Alaskan arctic field sites during summer 2014. Micro-topographic position in the landscape is indicated by text labels beneath x axis. Bars are mean ± standard error for each date (n = 6 or 7)
Sedge tiller density was the greatest in tussock sedges, and intermediate in dry graminoid and wet sedge communities. Sedges were not present in moss-lichen (Barrow-BEO) or moss only (Ivotuk) communities, and were only sparse in moss-shrub communities (0–573 tillers per m2 at Atqasuk and Ivotuk) (Table 1). Sedge height was the greatest in the wet sedge communities [Barrow: 17.6 ± 3.2 cm (n = 23); Atqasuk: 31.1 ± 5.9 cm (n = 7); Ivotuk: 37.7 ± 12.9 cm (n = 7) (Table 1)]. Highest DOC values ranged from between 26.1–127.1 mg/l, with the largest being found in the troughs at Barrow-BEO and lowest in the pools at Atqasuk (both wet sedge vegetation).
Spatial and Temporal Variability in CH4 Fluxes
Across all sites, the average CH4-C emissions were highest for wet sedge (1.68 ± 2.02 mg CH4-C m−2 h−1). Emissions were low from all other vegetation types: moss-lichen 0.00 ± 0.02 mg CH4-C m−2 h−1, moss-shrub 0.06 ± 0.15 mg CH4-C m−2 h−1, dry graminoid 0.10 ± 0.33 mg CH4-C m−2 h−1, moss only 0.25 ± 0.27 mg CH4-C m−2 h−1 and tussock sedge 0.46 ± 0.76 mg CH4-C m−2 h−1 (Figure 6A).
CH4 emissions were the highest in locations where the water table was either above or at the surface of the soil (Figure 6B). However, in tussock sedge plots, where the water table is found deep below the surface, substantial CH4 fluxes were recorded mid-late growing season (Figure 6A, B). Further, CH4 fluxes continued to increase throughout the season in the pools at Atqasuk, even when water table level dropped below the surface.
Controls of CH4 Fluxes
The multiple linear regression model explained 58% of the variability in CH4 fluxes \( \left( {R^{ 2}_{{{\text{GLMM}}({\text{m}})}} = \, 0. 5 8} \right) \). The most important variables identified were sedge height (Table 2; Figure 7A) and soil moisture (Table 2; Figure 7B), with greater sedge height and soil moisture increasing CH4 fluxes (Figure 7). The squared term of soil moisture was also significant, suggesting a curved relationship between soil moisture and CH4 emissions (Table 2).
Partial residual plots (isolating the relationship between CH4 flux and an explanatory variable, while the other environmental variables are held constant) for all significant environmental/vegetation variables identified in a multiple regression model A sedge height B soil moisture C water table height and D interaction between water table height and moss cover (high = where water table was above the surface of the soil, low = where water table was below the surface of the soil and mean = the average water table height across all four sites)
There was also a significant effect of water table height on CH4 fluxes across all four sites (Table 2; Figure 7C) and an interaction between water table height and moss cover (Table 2; Figure 7D). The relationship between moss cover and CH4 flux becomes increasingly negative as the water table decreases. When water table depth is one standard deviation above the mean value (+3.2 cm) and at the mean value (−8.7 cm), there is no significant relationship between moss cover and CH4 fluxes (P = 0.445, P = 0.063, respectively). At one standard deviation below the mean (−20.6 cm), there is a highly significant negative relationship between moss cover and CH4 flux (P = 0.001). There was no significant relationship between GPP and CH4 (Figure S3), as opposed to the significant relationship found during the previous year (Figure 8D).
Top panel this study A ‘dry’ and B ‘wet’ plots showing relationship between GPP and CH4 flux during 2014 measurement period at Barrow-BEO/Barrow-BES. Bottom panel McEwing and others C ‘dry’ and D ‘wet’ plots showing relationship between GPP and CH4 flux during 2013 measurement period at Barrow-BEN/Barrow-BEO/Barrow-BES. ‘Dry’ is defined as a dry graminoid community. ‘Wet’ is defined as a wet sedge community. See Table 1 for species composition
Following factor reduction in the second, a simplified multiple regression of just three categories was found to be adequate to describe the initial six vegetation types (Figure 3); one category including the wet sedge, one including tussock sedge types, and the other grouping the remaining four vegetation types of moss-lichen, moss-shrub, moss-only and dry graminoid. A much-simplified model including only these three vegetation types explained a considerable 54% \( \left( {R^{ 2}_{{{\text{GLMM}}({\text{m}})}} = \, 0. 5 4} \right) \) of variation in CH4 flux, with an additional 4% explained by soil moisture, a further 1% by sedge height and water table making no further improvement on the predictive power of the model.
Discussion
In this study, we show that vegetation was the dominant variable explaining the spatial heterogeneity of CH4 fluxes across a variety of tundra types across multiple vegetation communities, environmental conditions and geographic locations. Wet sedge communities appeared to dominate the CH4 emissions over the landscape, with other vegetation types contributing to much lower emission rates. Our findings demonstrate the importance of vegetation composition as an integrated measure of conditions relating to CH4 fluxes. Because of this, even a simplified vegetation classification using just three classes (Figure 3) was able to explain almost as much variation in CH4 fluxes (54%) as a model including multiple biotic and environmental drivers (58%). These findings pave the way for simplification of upscaling of CH4 fluxes using remote sensing, and thus improved prediction of CH4 fluxes in complex arctic landscapes by direct comparison of upscale flux data and model outputs. CH4 emission models are still limited by inadequate inclusion of the important controls on CH4 production, consumption and transport, as well as large errors in emission estimates from spatially heterogeneous landscapes (such as the ecosystems presented here) (Bridgham and others 2013).
Better characterisation of vegetation communities (most importantly sedge distribution and percent cover) can help inform process-based CH4 emission models (through understanding of plant-mediated transport emissions, for example) (Petrescu and others 2010) and ultimately improve model estimates. Direct comparison of modelled and measured fluxes has been limited by their different temporal and spatial scales. Our work provides a solution to at least partially reconcile the different spatial scales of model outputs and measured fluxes. Crucially, we show there is no relationship between instantaneous GPP and CH4 emissions, suggesting that CH4 fluxes across Arctic tundra are not always production limited. This strongly suggests that increasing primary productivity under elevated CO2 and a warming climate (Melton and others 2013) may not necessarily stimulate CH4 fluxes, in contrast to the assumptions of most existing modelling studies.
A similar study that measured GPP in two of the four field sites investigated here (Barrow-BEO, Barrow-BES) and a third site in Barrow (Barrow-BEN) McEwing and others 2015) found GPP to be a significant control on CH4 emissions in contrast to the results presented her, which found no significant relationship between GPP and CH4. Analysis of the dataset from (McEwing and others 2015) (Figure 8) show that both GPP and CH4 values at Barrow-BEO/Barrow-BES were larger in 2013 (GPP data from Ivotuk were not included in the analysis of McEwing and others 2015) than we report here for 2014. As vegetation community composition does not vary substantially between years, probably the different environmental conditions between 2013 (when McEwing and others 2015 study was performed) and 2014 (when the current study was undertaken) are responsible for this different result. 2013 was warmer than average (especially in Barrow), with the average air temperature ranging from 10.9°C in Barrow (with a maximum air temperature of 21.8°C on the 10th July 2013) compared to an average air temperature of 3.2°C in Barrow-BEO and Barrow-BES (with maximum of 16.1 on the 16th July 2014) during 2014. This resulted in an early snow-melt, and might have resulted in a more important role of plant productivity in stimulating CH4 fluxes. Similarly, a later than usual spring snow-melt in 2014 may have contributed to lower GPP values and the lack of a GPP relationship with CH4 production (D. Zona, unpublished data).
CH4 Flux Rates
The CH4 flux rates reported in this study were comparable with previous studies in similar Arctic sites (Wagner and others 2003; Kutzbach and others 2004; Zona and others 2009; Sturtevant and Oechel 2013). However, in contrast to these studies, we showed CH4 uptake in drier micro-topographic positions, which, although presenting low rates, may be important where these communities cover large areas (Hartley and others 2015).
Environmental Controls on CH4 Fluxes
As expected, in a multiple regression model, the most important environmental controls on CH4 flux were soil moisture, water table and sedge height, across all vegetation types, micro-topographic and geographic locations.
The importance of soil moisture and water table as a predictor of CH4 flux is consistent with many other studies (Zona and others 2009; Parmentier and others 2011; Mastepanov and others 2013; Olefeldt and others 2013; Sturtevant and others 2012). Soil moisture and position of the water table are linked, together dictating the volume of anaerobic and aerobic soil available in which methanogenesis and methanotrophy take place (van Huissteden and others 2005; Klapstein and others 2014; McCalley and others 2014). The polynomial relationship between soil moisture and CH4 emissions (Figure 7B) could be due to higher CH4 fluxes being present in ‘dry’ tussock sedge locations, where the water table is low within the soil column. On the other hand, soil moisture might also have an indirect control on CH4 emissions through its influence on vegetation type, because soil moisture was the most important factor explaining vegetation type in the NMDS ordination.
Sedge height has been highlighted as an important control on CH4 flux within the Barrow site (von Fischer and others 2010) and here we suggest that its importance holds across a broad geographical scale, and across a wide variety of vegetation types. Sedge height may play a pivotal role in controlling CH4 emissions because larger plants have a larger root system which may increase opportunities for transport of CH4 produced deeper in the most anoxic soil layers (Christensen and others 2000; von Fischer and others 2010). Furthermore, larger plants may result in more potential for CH4 release, as the location where CH4 may exit can be found along the length of the stem (Kelker and Chanton 1997; Juutinen and others 2003). Consequently, if the plant is located in an area submerged in water, the CH4 has more opportunity to be released through the part of the plant that is not covered by water (Kelker and Chanton 1997; Noyce and others 2013).
Despite the clear importance of factors such as soil moisture, sedge height, and water table, surprisingly, in the regression analysis, the more complex model (including soil moisture, sedge height, and water table) had very similar explanatory power (58%) compared to a very simplified model containing just one predictor—a three-class-vegetation type (which explained 54% of variation in CH4 flux). This finding likely arises from the capacity of vegetation to act as an integrator of many other environmental variables that also control CH4 flux, including the strong control that these variables have on vegetation type. For instance, there is a strong control of moisture on the vegetation present in these ecosystem (soil moisture status being the strongest determinant of above-ground vegetation communities across all four sites) (Figure 4B), and the importance of CH4 transport by sedges is evident in the much larger fluxes from sedge and tussock versus moss-only plant communities. As further model validation, the inclusion of the (McEwing and others 2015) dataset highlights the robustness of our model, with the simplified vegetation category model explaining 55% of variation in CH4 fluxes (model explained 54% of the variance in CH4 flux in our dataset alone), while still explaining 42% of the variance when using just the McEwing and other datasets. This showed that the model was consistent across years, and under very different environmental conditions.
Contrary to other studies, we showed no relationship between GPP and CH4 fluxes (Whiting and Chanton 1992, 1993; Harozono and others 2006; Lai and others 2014; McEwing and others 2015). This result is consistent with a lack of correlation between CH4 emissions and DOC. Thus, we suggest that CH4 production in these ecosystems is not usually limited by C input, consistent with ecosystem scale results from these sites (Zona and others 2009; Sturtevant and Oechel 2013), and that vegetation type is more likely to be a proxy for CH4 transport to the atmosphere. However, a stimulation of CH4 emissions might occur with higher plant productivity during particularly warm summers as reported by McEwing and others (2015). This is due to the close relationship between above ground vegetation communities and the soil moisture status below ground. If land cover can be defined into known vegetation communities, potential CH4 flux can be assumed by knowing whether species with a transport capability are present or not. We do however acknowledge that strong relationships between CH4 and GPP have been found previously (Harozono and others 2006; Lai and others 2014), which may be due to overall higher (cumulative GPP) productivity across a longer time scale. Our finding nonetheless emphasises that caution should be used when modelling an increase in CH4 emissions from increases in plant productivity (something which is characteristic of current CH4 models; Melton and others 2013). The lack of relationship between instantaneous GPP and CH4 fluxes in contrast to the strong importance of vegetation on CH4 fluxes in our study suggests a longer term influence of vascular plants on CH4 flux (von Fischer and Hedin 2007). Namely, identifying vegetation type can help understand potentially high CH4 emissions by whether the vegetation has the capacity to act as a conduit for CH4 release to the atmosphere, but not necessarily whether it has high GPP. Our study included a wide range of vegetation communities, some of which have substantial vegetation cover dominated by plant functional groups lacking aerenchymatous roots through which CH4 could be transported (Chapin and others 1996). As such, these communities may be expected to have higher GPP values but low CH4 fluxes. Therefore, we also examined instantaneous GPP–CH4 flux relationships with wet sedge-dominated vegetation only. Within wet sedge communities, we found only a very weak positive relationship between GPP and CH4 (Figure 8A), suggesting that the influence of GPP on CH4 flux may occur indirectly over longer timescales (for example, through increasing total DOC inputs to the soil, increased CH4 production through recent plant photosynthates in the form of root exudates, Dorodnikov and others 2011; Bridgham and others 2013).
In this study, we have highlighted the importance of plant-mediated transport in drier locations (McEwing and others 2015). Where the water table was deep within the soil column (>40 cm), a CH4 flux which was comparable with those of saturated wet sedge communities was found in tussock sedges at both Atqasuk and Ivotuk. Tussock sedge, E. vaginatum, is known to contain aerenchyma (Ström and others 2003), in contrast with the species present in the adjacent inter-tussock areas. These results suggest that the inundation fraction used by most models to estimate CH4 emissions from tundra (Bohn and others 2015) is not sufficient in these ecosystems and that plant community must also be considered. Further, this highlights the importance of determining fractions of micro-habitats (tussocks) within single vegetation communities to improve flux estimates.
Neither pH, active layer depth, nor soil temperature influenced CH4 emissions across all vegetation types and field sites. pH did not significantly vary across all four field sites nor did it correlate with CH4 flux, consistent with other studies across Arctic tundra ecosystems (Ohtsuka and others 2006; Brummell and others 2012). This indicates that in these ecosystems, fluxes are not significantly influenced by how acidic or alkaline the soil may be. Again, this simplifies estimation and upscaling of CH4 fluxes.
Soil temperature is known to strongly control the microbial activity necessary for methanogenesis (Whalen and Reeburgh 1988; Sachs and others 2010); however, in this study, soil temperatures were reasonably similar between both dry and wet microtopographic positions. The lack of relationship between soil temperature and CH4 emissions within this study could be attributed to other environmental controls dominating CH4 production (Rask and others 2002), where the whole soil column is anoxic. Drier areas show an increase in both CH4 oxidation and methanotrophy with an increase in soil temperature (Kutzbach and others 2004; Olefeldt and others 2013). Most importantly, the relatively short sampling campaign did not allow the inclusion of substantial temperature change. Other factors co-varying with soil temperature such as for example soil moisture, were more important for the explaining the spatial variability in CH4 flux when measured.
Overall, the substantial explanatory power of a simplified model including three vegetation groups (wet sedge communities, tussock sedge communities, and the other combining moss-only, moss-shrub, moss-lichen and dry graminoid communities) indicates that some refinement is needed beyond just looking at the presence/absence of sedges. We show that when differences in growth form are accounted for and sedge communities are considered in the wider context of their plant community, it is still possible to formulate a simplified model to predict CH4 emissions.
The results of this study provide an important approach to simplifying upscaling CH4 fluxes across heterogeneous tundra landscapes from the plot scale to the landscape scale. Although plant communities have been used for spatial upscaling of CH4 fluxes previously, the extent of our study across different vegetation communities and along a large latitudinal transect has allowed us to identify the minimum key drivers and recommend a much more simplified model for estimating CH4 emissions across Arctic tundra landscapes.
References
Aiken LS, West SG. 1991. Multiple regression: testing and interpreting interactions. Newbury Park: SAGE.
Bartlett RJ, Ross DS. 1988. Colorimetric determination of oxidizable carbon in acid soil solutions. Soil Sci Soc Am 52:1119–92.
Billings WD, Peterson KM. 1980. Vegetational change and ice-wedge polygons through the thaw lake cycle in Arctic Alaska. Arct Alp Res 12(4):413–32.
Bockheim JG, Everett LR, Hinkel KM, Nelson FE, Brown J. 1999. Soil organic carbon storage and distribution in arctic tundra, Barrow, Alaska. Soil Sci Soc Am J 63(4):934–40.
Bockheim JG, Hinkel KM, Nelson FE. 2001. Soils of the Barrow region Alaska. Polar Geogr 25(3):163–81.
Bohn TJ, Melton JR, Ito A, Kleinen T, Spahni R, Stocker BD, Zhang B, Zhu X, Schroeder R, Glagolev MV, Maksyutov S, Brovkin V, Chen G, Denisov SN, Eliseev AV, Gallego-Sala A, McDonald KC, Rawlins MA, Riley WJ, Subin ZM, Tian H, Zhuang Q, Kaplan JO. 2015. WETCHIMP-WSL: intercomparison of wetland methane emissions models over West Siberia. Biogeosci Discuss 12:1907–73. doi:10.5194/bgd-12-1907-2015.
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q. 2013. Methane emissions from wetlands: biogeochemical, microbial, and modelling perspectives from local to global scales. Glob Chang Biol 19:1325–436. doi:10.1111/gcb.12131.
Brown J. 1967. Tundra soils formed over ice wedges northern Alaska. Soil Sci Soc Am Proc 31:686–91.
Brummell ME, Farrell RE, Siciliano SD. 2012. Greenhouse gas soil production and surface fluxes at a high arctic polar oasis. Soil Biol Biochem 52:1–12.
Bubier JL. 1995. The relationship of vegetation to CH4 emission and hydrochemical gradients in northern peatlands. J Ecol 83:403–20.
Bubier JL, Moore TR, Bellisario L, Comer NT, Crill PM. 1995. Ecological control on CH4 emissions from a northern peatland complex in the zone of discontinuous permafrost. Manitoba, Canada. Glob Biogeochem Cycles 9(4):455–70.
Chapin FSIII, Bret-Harte MS, Hobbie SE, Hailin Z. 1996. Plant functional types are predictors of transient responses of arctic vegetation to global change. J Veg Sci 7:347–58.
Chapin FSIII, Sturm M, Serreze MC, McFadden JP, Key JR, Lloyd AH, McGuire AD, Rupp TS, Lynch AH, Schimel JP, Berlinger J, Chapman WL, Epstein HE, Euskirchen ES, Hinzman LD, Jia G, Ping C-L, Tape KD, Thompson CDC, Walker DA, Welker JM. 2005. Role of land-surface changes in Arctic summer warming. Science 310:656–60.
Christensen TR. 1993. Methane emission from Arctic tundra. Biogeochemistry 21(2):117–39.
Christensen TR, Friborg T, Sommerkorn M. 2000. Trace gas exchange in a high Arctic valley 1: variations in CO2 and CH4 flux between tundra vegetation types. Glob Biogeochem Cycles 14:701–14.
Christensen TR, Ekberg A, Ström L, Mastepanov M. 2003. Factors controlling large scale variations in CH4 emissions from wetlands. Geophys Res Lett 30(7):10–13.
Christensen TR, Johansson T, Akerman HJ, Mastepanov M. 2004. Thawing sub-Arctic permafrost: rffects on vegetation and CH4 emissions. Geophys Res Lett. doi:10.1029/2003GL018680.
Crawley MJ. 2012. The R Book. Chichester: Wiley.
Dlugokencky EJ, Nisbet EG, Fisher R, Fisher D, Lowrt D. 2011. Global atmospheric CH4: budget, changes and dangers. Philos Trans Ser A Math, Phys Eng Sci 369(1934):2058–72.
Dorodnikov M, Knorr K-H, Kuzyakov Y, Wilmking M. 2011. Plant-mediated CH4 transport and contribution of photosynthates to methanogenesis at a boreal mire: a 14C pulse-labeling study. Biogeosciences 8:2365–75.
Edwards EJ, Moody A, Walker DA. 2000. Field data report of ATLAS grids and transects 1998–1999. Fairbanks: Alaska Geobotany Center. pp 1998–9.
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J, Prinn R, Raga GMS, Van Dorland R 2007. Changes in atmospheric constituents and in radiative forcing in: Climate Change: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, In: Solomon S, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, Eds. Cambridge University Press, Cambridge.
Fox AM, Huntley B, Lloyd CR, Williams M, Baxter R. 2008. Net ecosystem exchange over heterogenerous Arctic tundra, scaling between chamber and eddy covariance measurements. Glob Biogeochem Sci 22(2):1–15.
Harazono Y, Mano M, Miyata A, Yoshimoto M, Zulueta RC, Vourlitis GL, Kwon H, Oechel WC. 2006. Temporal and spatial differences of CH4 flux at Arctic tundra in Alaska. Mem Natl Inst Polar Res Spec Issue 59:79–95.
Hartley IP, Hill TC, Wade T, Clement RJ, Moncrieff JB, Prieto-Blanco A, Disney MI, Huntly B, Williams M, Howden NJK, Wookey PA, Baxter R. 2015. Quantifying landscape-level methane fluxes in subarctic Finland using a multi-scale approach. Glob Chang Biol. doi:10.1111/gcb.12975.
Hultén E. 1968. Flora of Alaska and neighboring territories. Stanford: Stanford University Press.
IPCC. 2013. IPCC climate Change 2013: The Physical Science Basis. Cambridge: Cambridge University Press.
Joabsson A, Christensen TR. 2001. CH4 emissions from wetlands and their relationship with vascular plants: an Arctic example. Glob Chang Biol 7:919–32.
Juutinen S, Alm J, Larmola T, Huttunen JT, Morero M, Saarnio S, Martikaenen PJ, Sivola J. 2003. Methane (CH4) release from littoral wetlands of Boreal lakes during an extended flooding period. Glob Chang Biol 9:413–24.
Kade A, Bret-Harte MS, Euskirchen ES, Edgar C, Fulweber RA. 2012. Upscaling of CO2 fluxes from heterogeneous tundra plant communities in Arctic Alaska. J Geophys Res 117(4):1–11.
Kelker D, Chanton J. 1997. The effect of clipping on methane missions from Carex. Biogeochemistry 39:37–44. doi:10.1023/A:1005866403120.
King JY, Reeburgh WS, Regli SK. 1998. CH4 emission and transport by Arctic sedges in Alaska: results of a vegetation removal experiment. J Geophys Res 103:29083–92.
King JY, Reeburgh WS, Thieler KK, Kling GW, Loya WM, Johnson LC, Nadelhoffer KJ. 2002. Pulse-labelling studies of carbon cycling in Arctic tundra ecosystems: The contribution of photosynthates to methane emissions. Glob Biogeochem Cycles. doi:10.1029/2001GB001456.
Klaptstein SJ, Turetsky MR, McGuire DA, Harden JW, Czimczik CI, Xu X, Chanton JP, Waddington JM. 2014. Controls on CH4 released through ebullition in peatlands affected by permafrost degradation. J Geophys Res 119(3):418–31.
Koelbener A, Strom L, Edwards PJ, Venterink HO. 2010. Plant species from mesotrophic wetlands cause relatively high CH4 emissions from peat soil. Plant Soil 326:147–58.
Komarkova V, Webber PJ. 1980. Two low Arctic vegetation maps near Atkasook Alaska. Arct Alp Res 12(4):447–72.
Kutzbach L, Wagner D, Pfeiffer E-M. 2004. Effects of microrelief and vegetation on CH4 emission from wet polygonal tundra, Lena Delta, Northern Siberia. Biogeochemistry 69:341–62.
Kwon H-J, Oechel WC, Zulueta RC, Hastings SJ. 2006. Effects of climate variability on carbon sequestration among adjacent wet sedge tundra and moist tussock tundra ecosystems. J Geophys Res 111(3):1–18.
Lai DYF, Roulet NT, Moore TR. 2014. The spatial and temporal relationships between CO2 and CH4 exchange in a temperate ombrotrophic Bog. Atmos Environ 89:249–59.
McCalley CK, Woodcroft BJ, Hodgkins SB, Wher RA, Kim E-H, Mondav R, Crill PM, Chanton JP, Rich VI, Tyson GW, Saleska SR. 2014. CH4 dynamics regulated by microbial community response to permafrost thaw. Nature 514(7523):478–81.
McEwing KR, Fisher JP, Zona D. 2015. Environmental and vegetation controls on the spatial variability of CH4 emission from wet-sedge and tussock tundra ecosystems in the Arctic. Plant and Soil 388:37–52. doi:10.1007/s11104-014-2377-1.
McGuire AD, Christensen TR, Hayes D, Heroult A, Euskirchen E, Kimball JS, Koven C, Lafleur P, Miller PA, Oechel W, Peylin P, Williams M, Yi Y. 2012. An assessment of the carbon balance of Arctic tundra: comparisons among observations, process models, and atmospheric inversions. Biogeosciences 9(8):3185–204.
Mastepanov M, Sigsgaard C, Tagesson T, Ström L, Tamstorf MP, Lund M, Christensen TR. 2013. Revisiting factors controlling CH4 emissions from high-Arctic tundra. Biogeosciences 10:5139–58.
Matthews E, Fung I. 1987. Methane emission from natural wetlands: Global distribution, area and environmental characteristics of sources. Glob Biogeochem Cycles 1(1):61–86.
Melton JR, Wania R, Hodson EL, Poulter B, Ringeval B, Spahni R, Bohn T, Avis CA, Beerling DJ, Chen G, Eliseev AV, Denisov SN, Hopcroft PO, Lettenmaier DP, Riley WJ, Singarayer JS, Subin ZM, Tian H, Zürcher S, Brovkin V, van Bodegon PM, Kleinen T, Yu ZC, Kaplan JO. 2013. Present state of global wetland extent and wetland methane modelling: conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 10:753–88.
Michaelson GJ, Ping CL. 2003. Soil organic carbon and CO2 respiration at subzero temperature in soils of Arctic Alaska. J Geophys Res. doi:10.1029/2001JD000920.
Nakagawa S, Schielzeth H. 2013. A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–42.
Neff JC, Hooper DU. 2002. Vegetation and climate controls on potential CO2, DOC and DON production in northern latitude soils. Glob Chang Biol 8:872–84.
Noyce GL, Varner RK, Bubier JL, Frolking S. 2013. Effect of Carex rostrata on seasonal and interannual variability in peatland CH4 emissions. J Geophys Res 119(1):24–34.
Oechel WC, Vourlitis GL, Hastings SJ, Zulueta RC, Hinzman L, Kane D. 2000. Acclimation of ecosystem CO2 exchange in the Alaskan Arctic in response to decadal climate warming. Lett Nat 406:978–81.
Oechel WC, Laskowski CA, Burba G, Gioli B, Kalhjori AA. 2014. Annual patterns and budget of CO2 flux in an Arctic tussock tundra ecosystem. J Geophys Res. doi:10.1002/2013JG002433.
Ohtsuka T, Adachi M, Uchida M, Nakatsubo T. 2006. Relationships between vegetation types and soil properties along a topographical gradient on the northern coast of the Brøgger Peninsula Svalbard. Polar Biosci 19:63–72.
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Gavin L, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2013. vegan: Community Ecology Package. R package version 2.0-10. http://CRAN.R-project.org/package=vegan.
Olefeldt D, Turetsky MR, Crill PM, McGuire AD. 2013. Environmental and physical controls on northern terrestrial CH4 emissions across permafrost zones. Glob Chang Biol 19(2):589–603.
Olivas PC, Oberbauer SF, Tweedie CE, Oechel WC, Kuchy A. 2010. Responses of CO2 flux components of Alaskan Coastal Plain tundra to shifts in water table. J Geophys Res 115(4):1–13.
Parmentier FJW, van Huissteden J, van der Molen MK, Schaepman-Strub G, Karsanaev SA, Maximov TC, Dolman AJ. 2011. Spatial and temporal dynamics in eddy covariance observations of CH4 fluxes at a tundra site in northeastern Siberia. J Geophys Res 116(3):1–14.
Petrescu AMR, van Beek LPH, van Huissteden J, Prigent C, Sachs T, Corradi CAR, Parmentier FJW, Dolman AJ. 2010. Modelling regional CH4 emissions of boreal and arctic wetlands. Glob Biogeochem Cycles 24(4):1–12.
Pihlatie MK, Christiansen JR, Aaltonen H, Korhonen JFJ, Nordbo A, Rasilo T, Benanti G, Giebles M, Helmy M, Sheehy J, Jones S, Juszczack R, Klefoth R, Lobo-do-Vale R, Rosa AP, Schreiber P, Serca D, Vicca S, Wolf B, Pumpanen J. 2013. Comparison of static chambers to measure CH4 emissions from soils. Agric For Meteorol 171–172:124–36.
R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: http://www.R-project.org/.
Rask H, Schoenau J, Anderson D. 2002. Factors influencing methane flux from a boreal forest wetland in Saskatechewan Canada. Soil Biol Biochem 34(4):435–43.
Raynolds MK, Walker DA, Maier HA. 2005. Plant community-level mapping of Arctic Alaska based on the Circumpolar Arctic Vegetation Map. Phytocoenologia 35(4):821–48.
Raynolds MA, Walker DA, Maier, HA. 2006. Alaska Arctic Tundra Vegetation Map. Scale 1:4,000,000. Conservation of Arctic Flora and Fauna (CAFF) Map No. 2, U.S. Fish and Wildlife Service, Anchorage, Alaska.
Sachs T, Giebels M, Boike J, Kutzbach L. 2010. Environmental controls on CH4 emission from polygonal tundra on the microsite scale in the Lena River Delta, Siberia. Glob Chang Biol 16:3096–110.
Schimel JP. 1995. Plant transport and CH4 production as controls on CH4 flux from Arctic wet meadow tundra. Biogeochemistry 28:183–200.
Ström L, Ekberg A, Mastepanov M, Christensen TR. 2003. The effect of vascular plants on carbon turnover and CH4 emissions from a tundra wetland. Glob Chang Biol 9:1185–92.
Sturtevant CS, Oechel WC, Zona D, Kim Y, Emerson CE. 2012. Soil moisture control over autumn season CH4 flux, Arctic Coastal Plain of Alaska. Biogeosciences 9:1423–40.
Sturtevant CS, Oechel WC. 2013. Spatial variation in landscape-level CO2 and CH4 fluxes from arctic coastal tundra: influence from vegetation, wetness and the thaw lake cycle. Glob Chang Biol 19:2853–66.
Torn MS, Chapin FSIII. 1993. Environmental and biotic controls over CH4 flux from Arctic Tundra. Chemosphere 26(1–4):357–68.
USDA, NRCS. 2014. The PLANTS Database URL: http://plants.usda.gov, 13 August 2014. National Plant Data Team, Greensboro, NC 27401-4901 USA.
Vitt DH, Marsh JE, Bovey RB. 1988. Mosses, Lichens, and Ferns of Northwest North America. Vancouver: Lone Pine Publishing.
Van Huissteden J, Maximov TC, Dolman AJ. 2005. High CH4 flux from an arctic floodplain (Indigirka lowlands, eastern Siberia). J Geophys Res. doi:10.1029/2005JG000010.
von Fischer JC, Rhew RC, Ames GM, Fosdick BK, von Fischer PE. 2010. Vegetation height and other controls of spatial variability in CH4 emissions from the Arctic coastal tundra at Barrow Alaska. J Geophys Res 115(3):1–11.
von Fischer J, Hedin L. 2007. Controls on soil methane fluxes: tests of biophysical mechanisms using stable isotope tracers. Glob Biogeochem Cycles 21(2):1–9.
Wagner D, Kobabe S, Pfeiffer E-M, Hubberten H-W. 2003. Microbial controls on methane fluxes from a polygonal tundra of the lena delta Siberia. Permafr Periglac Process 14:173–85.
Walker DA, Binnian E, Evans BM, Lederer ND, Nordstrand E, Webber PJ. 1989. Terrain, vegetation and landscape evolution of the R4D research site, Brooks Range Foothills Alaska. Holarct Ecol 12:238–61.
Webber P. 1978. Spatial and temporal variation in the vegetation and its productivity, Barrow, Alaska. In: Tieszen LL, Ed. Vegetation and production ecology of an Alaskan Arctic Tundra. New York: Springer. p 37–112.
Whalen SC, Reeburgh W. 1988. A methane time flux series for tundra environments. Glob Biogeochem Cycles 2:399–409.
Whiting GJ, Chanton JP. 1992. Plant dependent CH4 emission in a subarctic Canadian fen. Glob Biogeochem Cycles 6:225–31.
Whiting GJ, Chanton JP. 1993. Primary production control of CH4 emission from wetlands. Nature 364:794–5.
Zona D, Oechel WC, Kochendorfer J, Paw KT, Salyuk UAN, Olivas PC, Oberbauer SF, Lipson DA. 2009. CH4 fluxes during the initiation of a large-scale water table manipulation experiment in the Alaskan Arctic tundra. Glob Biogeochem Cycles 23(2):1–11.
Zona D, Gioli B, Commane R, Lindaas J, Wofsy SC, Miller CE, Dinardo SJ, Dengel S, Sweeney C, Karion A, Chang RY-W, Henderson JM, Murphy PC, Goodrich JP, Moreaux V, Liljedahl A, Watts JD, Kimball JS, Lipson DA, Oechel WC. 2016. Cold season emissions dominate the Arctic tundra methane budget. Proc Natl Acad Sci 113(1):40–5.
Acknowledgements
This work was funded by the Office of Polar Programs of the National Science Foundation (NSF) awarded to DZ (award number 1204263) and Carbon in Arctic Reservoirs Vulnerability Experiment (CARVE), an Earth Ventures (EV-1) investigation, under contract with the National Aeronautics and Space Administration. Additional funding was provided by a Royal Society International Exchange awarded to DZ (2013/R1), and a NERC Arctic Research Programme, CYCLOPS Grant (NE/K00025X/1) to GKP. SJD is supported by a NERC PhD studentship. This research was conducted on land owned by the Ukpeagvik Inupiat Corporation (UIC) and the Arctic Slope Regional Corporation (ASRC). We would like to thank the Global Change Research Group at San Diego State University in particular Patrick Murphy and UMIAQ and UIC for logistical support, Jennifer Watts, Ali Hoy, and Owen Hayman for the help in the field. We thank Skip Walker and Martha Raynolds at the Alaskan Geobotany Centre, University of Fairbanks for the use of the Alaskan vegetation map and Cole Kellner at the Polar Geospatial Centre, University of Minnesota. We also thank the R development core team. We thank the anonymous referees and the editor for their constructive comments on our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contribution DZ, WCO, and GKP secured the funding, DZ, WCO, GKP, SJD, and VLS designed the study, SJD and VLS performed the research, SJD and VLS analysed the data with input from DZ and GKP, RW collected and analysed the DOC data, JPF helped with statistical analyses, SJD, VLS, GKP, WCO and DZ wrote the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Davidson, S.J., Sloan, V.L., Phoenix, G.K. et al. Vegetation Type Dominates the Spatial Variability in CH4 Emissions Across Multiple Arctic Tundra Landscapes. Ecosystems 19, 1116–1132 (2016). https://doi.org/10.1007/s10021-016-9991-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-016-9991-0