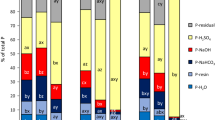
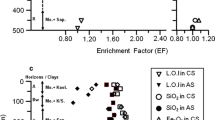
Abstract
The chemical and mineralogical compositions of three tropical soils, before and after permeation with 19–24 pore volumes of acid mine drainage (AMD), were assessed using X-ray diffraction and chemical analyses, in order to consider their potential value as clay liners. After permeation the CEC of one soil (Soil K) was reduced, partly due to structural modification of smectite by AMD. Conversely, the other soils (Soils A and H) showed increased CEC values due to structural changes in mixed layer vermiculite minerals, resulting in the formation of vermiculite as a separate phase in the soils. The specific surfaces of the soils were reduced. AMD caused changes in the variable charge properties of the soils due to the composite effects of soil pH and organic matter reduction and the changes in composition of exchangeable ions. Dolomite, gibbsite, diaspore, magnesioferrite and hydroxy apatite were dissolved from the soils. Chlorite was mildly altered but kaolinite was structurally resistant to AMD attack. Jarosites were, however, formed in all the soils. It was concluded that the tropical soils studied could be effective sinks for zinc and nickel from AMD, but that Soils A and H would be the most desirable clay liners for acid mine waste containment due to their low hydraulic conductivity, high sorptive capacity and compatibility with AMD.
Résumé
Les compositions chimiques et minéralogiques de trois sols tropicaux, avant et après percolation en essai de drainage acide minier (AMD), ont été étudiées à partir d’essais de diffractométrie RX et d¹analyses chimiques, afin de préciser leur utilisation possible comme revêtement argileux. Après percolation, la CEC de l’un des sols a diminué, en partie du fait de modifications structurales d’une smectite par le drainage acide. A l’inverse, la CEC des autres sols a augmenté du fait de changement de structure de minéraux argileux interstratifiés à base de vermiculite, correspondant à la formation de vermiculite dans ces sols. Les surfaces spécifiques de ces sols ont diminué. Le drainage acide a causé des modifications du complexe d’adsorption de ces sols du fait des effets combinés du pH du sol, de la matière organique et des ions échangeables. De la dolomite, de la gibbsite, du diaspore, de la magnésioferrite et de l’hydroxy apatite ont été dissous dans ces sols. La chlorite a été légèrement altérée mais la kaolinite a résisté aux attaques acides, de par sa structure. De la jarosite s’est cependant formée dans tous ces sols. On a conclu que les sols tropicaux étudiés pouvaient constituer des puits pour le zinc et le nickel après attaque acide. Les sols A et H seraient les plus adaptés comme recouvrement argileux pour des digues de confinement de déchets issus de drainage acide, du fait de leur faible conductivité hydraulique, de leur forte capacité d’adsorption et de leur compatibilité avec le drainage acide.














Similar content being viewed by others
References
Ahn PM (1970) West African soils, 3rd edn. Oxford University, University Press, London, 332pp
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2/PRODEFA2, a geochemical assessment model for environmental systems—version 3.0 user’s manual: U.S. Environmental Protection Agency Report EPA/600/3-91/021106 p
American Society for Testing and Materials (2002) Annual book of ASTM standards. Section 4 Construction—volume 04.08 soil and rock (I): D 420-D 5779. ASTM International, West Conshohocken, PA, 1672pp
Bloom PR, Erich MS (1987) Effect of solution composition on the rate and mechanism of gibbsite dissolution in acid solutions. Soil Sci Soc Am J 51:1131–1136
Brammer H (1962) Ghana soils. In: Wills JB (ed) Agriculture and land use in Ghana. Oxford University Press, London, pp 88–126
Brindley GW, Brown G (1989) Crystal structures of clay minerals and their X-ray identification. Mineralogical Society Monograph No. 5, Mineralogical Society, London
Brown G (1961) The X-ray identification and crystal structures of clay minerals. Mineralogical Society, London
Brown G, Brindley GW (1989) X-ray diffraction procedures for clay identification. In: Brindley GW, Brown G (eds) Crystal structures of clay minerals and their X-ray identification. Mineralogical Society, London, pp 305–359
Building and Road Research Institute and Lyon Associates Inc. (1971) Laterites and lateritic soils and other problem soils of Africa. An engineering study for USAID, AID/csd-2164
Carnicelli S, Mirabella A, Cecchini G, Sanesi G (1997) Weathering of chlorite to a low-charge expandable mineral in a spodosol on the Apennine Mountains, Italy. Clays Clay Minerals 45(1):28–41
Caroll D (1974) Clay minerals: a guide to their X-ray identification. The Geological Society of America, Special paper, p 126
Chao TT, Zhou L (1983) Extraction techniques for selective dissolution of amorphous iron oxides from soils and sediments. Am J Soil Sci Soc 47:225–232
Dixon JB, Weed CB (1977) Minerals in soil environments. Soil Science Society of America, Madison
Dremanis A (1962) Quantitative gasometric determination of calcite and dolomite by using Chittick Apparatus. J Sediment Petrol 32(3):520–529
Drouet C, Pass KL, Baron D, Draucker S, Navrotsky A. (2004) Thermochemistry of jarosite–alunite and natrojarosite–natroalunite solid solutions. Geochemica Cosmochimica Acta 68(10):2197–2205
Frempong EM, Yanful EK (2004) The effectiveness of some tropical soil liners in the retention of heavy metals released from ARD. In: Proceedings of 57th Canadian geotechnical conference, Quebec City, Canada (Oct. 24–27, 2004), Session 7D, pp 1–8
Goodyear J, Duffin WJ (1954) Identification of plagioclase feldspars by the X-ray powder methods. Mineral Mag 30:306–326
Gray NF (1997) Environmental impact and remediation of acid mine drainage: a management problem. Environ Geol 30 (1/2):358–361
Grim RE (1968) Clay mineralogy. McGraw Hill, New York
Hamer R, Graham RC, Amrhein C, Bozhiloc KN (2003) Dissolution of rippidolite (Mg, Fe-chlorite) in organic and inorganic solutions. Soil Sci Soc Am J 67:654–661
Hartemink AE (2004) Soils of the tropics. Geoderma 123:373–375
Hughes RE, Moore DM, Glass HD (1994) Qualiltative and quantitative analysis of clay mineral in soils. In: Amonette JE, Zelazny LW (eds) Quantitative methods in soil mineralogy. Soil Science Society of America (SSSA) Miscellaneous Publication. SSSA Inc., Madison, pp 330–359
Iwata S (1995) Interaction between particles through water. In: Iwate S, Tabuchi T, Warkentin BP (eds) Soil–water interactions: mechanisms and applications. Marcel Dekker Inc., New York, pp 154–228
Kahle M, Kleber M, Jahn R (2002) Review of XRD-based quantitative analyses of clay minerals in soils: the suitability of mineral intensity factors. Geoderma 109:191–205
Kalinowski BE, Schweda P (1996) Kinetics of muskovite, phlogopite, and biotite dissolution and alteration at pH 1–4, room temperature. Geochim Cosmochim Acta 60:367–385
Kashir M, Yanful EK (1997). A flow pump system for assessing clay barrier-permeant compatibility. Geotech Test J 20(2):179–190
Kashir M, Yanful EK (2000) Compatibility of slurry wall backfill soils with acid mine drainage. Adv Environ Res 4(3):251–268
Kashir M, Yanful EK (2001) Hydraulic conductivity of bentonite permeated with acid mine drainage. Can Geotech J 32(5):1034–1048
MacEwan DM (1950) Some notes on the recording and interpretation of X-ray diagrams of clay soils. J Soil Sci 1:90–103
Manzano M, Ayora C, Domenech C, Navarette P, Garralon A, Turrero M-J (1999) The impact of the Aznalcollar mine-tailing spill on groundwater. Sci Total Environ 242(1–3):189–209
Martin RT (1955) Glycol retention analysis. Proc Soil Sci Soc Am 19(2):160–164
Mendoça RGM, Barbosa MC Castro FJCO (2002) Evaluation of ions retention capacity of a residual soil of Rio de Janeiro, Brazil. In: De Mello G, Almeida M (eds) Proceedings of 4th international congress on environmental geotechnics, Rio de Janeiro, Brazil 1:439–446
Mitchell JK (1993) Fundamentals of soil behaviour, 2nd edn. Wiley, New York
Moore DM, Reynolds RC (1997) X-ray diffraction and the identification and analysis of minerals. Oxford University Press Inc., New York
Murray HH (1951) The structure of kaolinite and its relation to acid treatment. PhD Thesis, University of Illinois
Nordstrom DK (1982) Aqueous pyrite oxidation and the subsequent formation of secondary iron minerals. In: Kittrick JA, Fanning, DS, Hossner LR (eds) Acid sulfate weathering. Soil Science Society of America, Madison, Special Publication, #10, pp 37–56
Omidi GH, Thomas JC, Brown KW (1995) Effect of desiccation cracking on the hydraulic conductivity of a compacted clay liner. Water Air Soil Pollut 89:91–10
Perrin RMS (1971) The clay mineralogy of British sediments. Mineralogical Society, London
Rich CI (1968) Hydroxy interlayers in expansible layer silicates. Clays Clay Miner 16:15–30
Ridley MK, Wesolowski DJ, Palmer DA, Benezeth P, Kettler RM (1997) Effect of sulphate on the release rate of Al3+ from gibbsite in low-temperature acidic waters. Environ Sci Technol 31:1922–1925
Ross GJ (1968) Structural decomposition of orthochlorite during its acid dissolution. Can Mineral 9:522–530
Ross GJ (1969) Acid dissolution of chlorites: release of magnesium, iron and aluminium and mode of attack. Clays Clay Minerals 17:347–354
Ross GJ, Wang C (1993) Extractable Al, Fe, Mn, and Si. In: Carter MR (eds) Soil sampling and methods of analysis. Canadian Society of Soil Science, Lewis Publishers
Ross GJ, Wang C, Schuppli PA (1985) Hydroxylamine and ammonium oxalate solutions as extractants for Fe and Al from soil. Am J Soil Sci Soc 49:783–785
Sawhney BJ (1977) Interstratification in layer silicates. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Science Society of America, Madison, pp 405–434
Schwertmann U, Taylor RM (1977) Iron oxides. In: Dixon JB, Weed SB (eds) Minerals in soil environments. Soil Science Society of America, Madison, pp 145–172
Sposito G (1989) The chemistry of soils. Oxford University Press, New York
Šucha V, Środoń J, Clauer N, Elsass F, Elberl DD, Kraus I, Madejová J (2001) Weathering of smectite and illite–smectite under temperate climatic conditions. Clays Clay Minerals 36:403–419
Suraj G, Iyer CSP, Lalithambika M (1998) Adsorption of cadmium and copper by modified kaolinites. Appl Clay Sci 13:293–306
United States Department of Agriculture (1996) Soil survey laboratory methods manual. Soil survey investigations Report No. 42 Version 3.0. National Soil Survey Center, Natural Resources Conservation Service, USDA, January 1996
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 34:29–38
Wang C, Schuppli PA, Ross GJ (1987) A comparison of hydroxylamine and ammonium oxalate solutions as extractants for Al, Fe and Si from Spodosols and Spodosol-like soils in Canada. Geoderma 40:345–355
Welch SA, Ullman WJ (1996) Feldspar dissolution in acidic and organic solutions: compositional and pH dependence of dissolution rate. Geochimica Cosmochimica Acta 60 (16):2939–2948
Yanful EK, Shikatani KS, Quirt DH (1995) Hydraulic conductivity of natural soils permeated with acid mine drainage. Can Geotechn J 32:624–646
Young A (1976) Tropical soils and soil surveys. Cambridge University Press, London
Acknowledgements
This work was made possible through scholarships given to E. M. Frempong by the Canadian Commonwealth Scholarship and Fellowship Program and the Council for Scientific and Industrial Research, Ghana and Discovery Grant awarded to E. K. Yanful by the Natural Sciences and Engineering Research Council, Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frempong, E.M., Yanful, E.K. Chemical and mineralogical transformations in three tropical soils due to permeation with acid mine drainage. Bull Eng Geol Environ 65, 253–271 (2006). https://doi.org/10.1007/s10064-005-0029-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10064-005-0029-7