Abstract
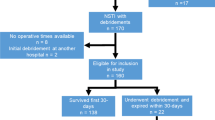
Necrotizing soft tissue infection (NSTI) due to group A Streptococcus (GAS) is a severe life-threatening microbial infection. The administration of adjunct clindamycin has been recommended in the treatment of NSTIs due to GAS. However, robust evidence regarding the clinical benefits of adjunct clindamycin in NSTI patients remains controversial. We aimed to investigate the association between early administration of adjunct clindamycin and in-hospital mortality in patients with NSTI attributed to GAS. The present study was a nationwide retrospective cohort study, using the Japanese Diagnosis Procedure Combination inpatient database focusing on the period between 2010 and 2018. Data was extracted on patients diagnosed with NSTI due to GAS. We compared patients who were administered clindamycin on the day of admission (clindamycin group) with those who were not (control group). A propensity score overlap weighting method was adopted to adjust the unbalanced backgrounds. The primary endpoint was in-hospital mortality and survival at 90 days after admission. We identified 404 eligible patients during the study period. After adjustment, patients in the clindamycin group were not significantly associated with reduced in-hospital mortality (19.2% vs. 17.5%; odds ratio, 1.11; 95% confidence interval, 0.59–2.09; p = 0.74) or improved survival at 90 days after admission (hazard ratio, 0.92; 95% confidence interval, 0.51–1.68; p = 0.80). In this retrospective study, early adjunct clindamycin does not appear to improve survival. Therefore, the present study questions the benefits of clindamycin as an adjunct to broad spectrum antibiotics in patients with NSTI due to GAS.


Similar content being viewed by others
Data availability
The Japanese Diagnosis Procedure Combination inpatient database.
Code availability
Stata/MP15 (Stata Corp, College Station, TX, USA)
Abbreviations
- NSTI:
-
Necrotizing soft tissue infection
- GAS:
-
Group A Streptococcus
- ICD-10:
-
International Classification of Diseases, Tenth Revision
- PS:
-
Propensity score
References
Hua C, Bosc R, Sbidian E, De Prost N, Hughes C, Jabre P et al (2018) Interventions for necrotizing soft tissue infections in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD011680.pub2
Madsen MB, Skrede S, Perner A, Arnell P, Nekludov M, Bruun T et al (2019) Patient’s characteristics and outcomes in necrotising soft-tissue infections: results from a Scandinavian, multicentre, prospective cohort study. Intensive Care Med 45:1241–1251. https://doi.org/10.1007/s00134-019-05730-x
Urbina T, Madsen MB, de Prost N (2020) Understanding necrotizing soft tissue infections in the intensive care unit. Intensive Care Med 46:1739–1742. https://doi.org/10.1007/s00134-020-06071-w
Peetermans M, de Prost N, Eckmann C, Norrby-Teglund A, Skrede S, De Waele JJ (2020) Necrotizing skin and soft-tissue infections in the intensive care unit. Clin Microbiol Infect 26:8–17. https://doi.org/10.1016/j.cmi.2019.06.031
Stevens DL, Bryant AE (2017) Necrotizing soft-tissue infections. N Engl J Med 377:2253–2265. https://doi.org/10.1056/NEJMra1600673
Kaul R, McGeer A, Low DE, Green K, Schwartz B, Simor AE et al (1997) Population-based surveillance for group A streptococcal necrotiziug fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Am J Med 103:18–24. https://doi.org/10.1016/S0002-9343(97)00160-5
Stevens DL (2000) Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med 51:271–288
Melissa Johnson (2021) Clindamycin: An overview. Up To Date. https://www.uptodate.com/contents/clindamycin-an-overview?search=clindamycin&source=search_result&selectedTitle=2~145&usage_type=default&display_rank=1. Accessed 13 October 2021
Yan S, Bohach GA, Stevens DL (1994) Persistent acylation of high-molecular-weight penicillin-binding proteins by penicillin induces the postantibiotic effect in Streptococcus pyogmes. J Infect Dis 170:609–614. https://doi.org/10.1093/infdis/170.3.609
Mascini EM, Jansze M, Schouls LM, Verhoef J, Van Dijk H (2001) Penicillin and clindamycin differentially inhibit the production of pyrogenic exotoxins A and B by group A streptococci. Int J Antimicrob Agents 18:395–398. https://doi.org/10.1016/S0924-8579(01)00413-7
Stevens DL, Bryant AE, Hackett SP (1995) Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis 20:S154–S157. https://doi.org/10.1093/clinids/20.Supplement_2.S154
Stevens DL, Yan S, Bryant AE (1993) Penicillin-binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J Infect Dis 167:1401–1405. https://doi.org/10.1093/infdis/167.6.1401
Gemmell CG, Peterson PK, Schmeling D, Kim Y, Mathews J, Wannamaker L et al (1981) Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J Clin Invest 67:1249–1256. https://doi.org/10.1172/JCI110152
Eagle H (1952) Experimental approach to the problem to treat ment failure with penicillin. Am J Med 175:389–399. https://doi.org/10.1111/j.0954-6820.1964.tb04669.x
Andreoni F, Zörcher C, Tarnutzer A, Schilcher K, Neff A, Keller N et al (2017) Clindamycin affects group a streptococcus virulence factors and improves clinical outcome. J Infect Dis 215:269–277. https://doi.org/10.1093/infdis/jiw229
Zimbelman J, Palmer A, Todd J (1999) Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 18:1096–1100. https://doi.org/10.1097/00006454-199912000-00014
Mulla ZD, Leaverton PE, Wiersma ST (2003) Invasive Group A Streptococcal Infections in Florida. South Med J 96:968–73. https://doi.org/10.1503/cmaj.060241
Carapetis JR, Jacoby P, Carville K, Ang SJJ, Curtis N, Andrews R (2014) Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group a streptococcal infections. Clin Infect Dis 59:358–365. https://doi.org/10.1093/cid/ciu304
Couture-Cossette A, Carignan A, Mercier A, Desruisseaux C, Valiquette L, Pépin J (2018) Secular trends in incidence of invasive betahemolytic streptococci and efficacy of adjunctive therapy in Quebec, Canada, 1996–2016. PLoS ONE 13:1–17. https://doi.org/10.1371/journal.pone.0206289
Babiker A, Li X, Lai YL, Strich JR, Warner S, Sarzynski S et al (2021) Effectiveness of adjunctive clindamycin in β-lactam antibiotic-treated patients with invasive β-haemolytic streptococcal infections in US hospitals: a retrospective multicentre cohort study. Lancet Infect Dis 3099:1–13. https://doi.org/10.1016/S1473-3099(20)30523-5
Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL et al (2014) Practice guidelines for the diagnosis and management of skin and soft tissue infections: update by the infectious diseases society of America. Clin Infect Dis 2014:59. https://doi.org/10.1093/cid/ciu296
Yasunaga H (2019) Real World Data in Japan: Chapter II The Diagnosis Procedure Combination Database. Ann Clin Epidemiol 1:76–79
Lundgren RS, Kramer CB, Rivara FP, Wang J, Heimbach DM, Gibran NS et al (2009) Influence of comorbidities and age on outcome following burn injury in older adults. J Burn Care Res 30:307–314. https://doi.org/10.1097/BCR.0b013e318198a416
Ono K, Wada K, Takahara T, Shirotani T (2007) Indications for computed tomography in patients with mild head injury. Neurol Med Chir (Tokyo) 47:291–297. https://doi.org/10.2176/nmc.47.291
Suissa S (2008) Immortal time bias in pharmacoepidemiology. Am J Epidemiol 167:492–499. https://doi.org/10.1093/aje/kwm324
Defining Adult Overwight and Obesity. https://www.cdc.gov/obesity/adult/defining.html. Accessed 21 Nov 2019
Desai RJ, Franklin JM (2019) Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 367:1–10. https://doi.org/10.1136/bmj.l5657
Thomas LE, Li F, Pencina MJ (2020) Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 323:2417–2418. https://doi.org/10.1001/jama.2020.7819
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107. https://doi.org/10.1002/sim
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424. https://doi.org/10.1080/00273171.2011.568786
Li F, Thomas LE (2019) Addressing extreme propensity scores via the overlap weights. Am J Epidemiol 188:250–257. https://doi.org/10.1093/aje/kwy201
Minami M, Kamimura T, Isaka M, Tatsuno I, Ohta M, Hasegawa T (2010) Clindamycin-induced CovS-mediated regulation of the production of virulent exoproteins streptolysin O, NAD glycohydrolase, and streptokinase in Streptococcus pyogenes. Antimicrob Agents Chemother 54:98–102. https://doi.org/10.1128/AAC.00804-09
Šmitran A, Vuković D, Opavski N, Gajić I, Marinković J, Božić L et al (2018) Influence of subinhibitory antibiotic concentration on streptococcus pyogenes adherence and biofilm production. Acta Microbiol Immunol Hung 65:229–240. https://doi.org/10.1556/030.65.2018.026
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007 and 20AA2005) and the Ministry of Education, Culture, Sports, Science, and Technology, Japan (20H03907)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The present study was approved by The University of Tokyo Institutional Review Board (approval number: 3501–1). Because the data are anonymous, the requirement for patient informed consent was waived.
Consent to participate
All authors directly participated in the planning, execution, or analysis of the study.
Consent for publication
The contents of this manuscript have not been copyrighted or published previously and are not under consideration for publication elsewhere. All authors have read and approved the final version submitted.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamada, S., Nakajima, M., Kaszynski, R.H. et al. Association between adjunct clindamycin and in-hospital mortality in patients with necrotizing soft tissue infection due to group A Streptococcus: a nationwide cohort study. Eur J Clin Microbiol Infect Dis 41, 263–270 (2022). https://doi.org/10.1007/s10096-021-04376-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-021-04376-2