Abstract
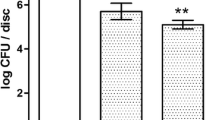
Effective decontamination of biofilm and bacterial toxins from the surface of dental implants is a yet unresolved issue. This study investigates the in vitro efficacy of photodynamic treatment (PDT) with methylene blue (MB) photoactivated with λ 635 nm diode laser and of λ 405 nm violet-blue LED phototreatment for the reduction of bacterial biofilm and lipopolysaccharide (LPS) adherent to titanium surface mimicking the bone-implant interface. Staphylococcus aureus biofilm grown on titanium discs with a moderately rough surface was subjected to either PDT (0.1% MB and λ 635 nm diode laser) or λ 405 nm LED phototreatment for 1 and 5 min. Bactericidal effect was evaluated by vital staining and residual colony-forming unit count. Biofilm and titanium surface morphology were analyzed by scanning electron microscopy (SEM). In parallel experiments, discs coated with Escherichia coli LPS were treated as above before seeding with RAW 264.7 macrophages to quantify LPS-driven inflammatory cell activation by measuring the enhanced generation of nitric oxide (NO). Both PDT and LED phototreatment induced a statistically significant (p < 0.05 or higher) reduction of viable bacteria, up to −99 and −98% (5 min), respectively. Moreover, besides bactericidal effect, PDT and LED phototreatment also inhibited LPS bioactivity, assayed as nitrite formation, up to −42%, thereby blunting host inflammatory response. Non-invasive phototherapy emerges as an attractive alternative in the treatment of peri-implantitis to reduce bacteria and LPS adherent to titanium implant surface without causing damage of surface microstructure. Its efficacy in the clinical setting remains to be investigated.




Similar content being viewed by others
References
Albrektsson T, Canullo L, Cochran D, De Bruyn H (2016) “Peri-implantitis”: a complication of a foreign body or a man-made “disease”. Facts and fiction. Clin Implant Dent Relat Res 18:840–849
Zitzmann NU, Berglundh T (2008) Definition and prevalence of peri-implant diseases. J Clin Periodontol 35:286–291
Tomasi C, Derks J (2012) Clinical research of peri-implant diseases: quality of reporting, case definitions and methods to study incidence, prevalence and risk factors of peri-implant diseases. J Clin Periodontol 12:207–223
Renvert S, Quirynen M (2015) Risk indicators for peri-implantitis. A narrative review. Clin Oral Implant Res 11:15–44
Bi Y, Seabold J, Kaar S, Ragab A, Goldberg V, Anderson J, Greenfield E (2001) Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res 16:2082–2091
Wataha J, Hanes P, Lockwood P (2001) Effect of lipopolysaccharide contamination on the attachment of osteoblast-like cells to titanium and titanium alloy in vitro. J Oral Implantol 27:174–179
Morra M, Cassinelli C, Bollati D, Cascardo G, Bellanda M (2015) Adherent endotoxin on dental implant surfaces: a reappraisal. J Oral Implantol 41:10–16
Persson GR, Renvert S (2014) Cluster of bacteria associated with peri-implantitis. Clin Implant Dent Relat Res 16:783–793
Harris LG, Richards RG (2004) Staphylococcus aureus adhesion to different treated titanium surfaces. J Mater Sci Mater Med 15:311–314
Teughels W, Van Assche N, Sliepen I, Quirynen M (2006) Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implant Res 17:68–81
Al-Ahmad A, Wiedmann-Al-Ahmad M, Fackler A, Follo M, Hellwig E, Bächle M, Hannig C, Han JS, Wolkewitz M, Kohal R (2013) In vivo study of the initial bacterial adhesion on different implant materials. Arch Oral Biol 58:1139–1147
Ferreira Ribeiro C, Cogo-Müller K, Franco GC, Silva-Concílio LR, Sampaio Campos M, de Mello RS, Claro Neves AC (2016) Initial oral biofilm formation on titanium implants with different surface treatments: an in vivo study. Arch Oral Biol 69:33–39
Karring ES, Stavropoulos A, Ellegaard B, Karring T (2005) Treatment of peri-implantitis by the Vector system. Clin Oral Implant Res 16:288–293
Renvert S, Lessem J, Dahlen G, Lindahl C, Svensson M (2006) Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: a randomized clinical trial. J Clin Periodontol 33:362–369
Al-Hashedi AA, Laurenti M, Benhamou V, Tamimi F (2016) Decontamination of titanium implants using physical methods. Clin Oral Implant Res. doi:10.1111/clr.12914
Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G (2001) Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implant Res 12:104–108
Chan Y, Lai CH (2003) Bactericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci 18:51–55
Wainwright M (2005) The development of phenothiazinium photosensitisers. Photodiagnosis Photodyn Ther 2:263–272
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther 1:279–293
Maclean M, MacGregor SJ, Anderson JG, Woolsey GA (2009) Inactivation of bacterial pathogens following exposure to light from a 405nm LED array. Appl Environ Microbiol 75:1932–1937
Fontana CR, Song X, Polymeri A, Goodson JM, Wang X, Soukos NS (2015) The effect of blue light on periodontal biofilm growth in vitro. Lasers Med Sci 30:2077–2086
Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, Doukas AG, Goodson JM (2005) Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother 49:1391–1396
Kurz J, Eberle F, Graumann T, Kaschel ME, Sähr A, Neumann F, Dalpke AH, Erdinger L (2011) Inactivation of LPS and RNase A on photocatalytically active surfaces. Chemosphere 84:1188–1193
Bornstein E (2004) Near-infrared dental diode lasers. Scientific and photobiologic principles and applications. Dent Today 23:102–108
Harrison JJ, Stremick CA, Turner RJ, Allan ND, Olson ME, Ceri H (2010) Microtiter susceptibility testing of microbes growing on peg lids: a miniaturized biofilm model for high-throughput screening. Nat Protoc 5:1236–1254
Giannelli M, Bani D, Tani A, Pini A, Margheri M, Zecchi-Orlandini S, Tonelli P, Formigli L (2009) In vitro evaluation of the effects of low-intensity Nd:YAG laser irradiation on the inflammatory reaction elicited by bacterial lipopolysaccharide adherent to titanium dental implants. J Periodontol 80:977–984
Giannelli M, Pini A, Formigli L, Bani D (2011) Comparative in vitro study among the effects of different laser and LED irradiation protocols and conventional chlorhexidine treatment for deactivation of bacterial lipopolysaccharide adherent to titanium surface. Photomed Laser Surg 29:573–580
Giannelli M, Landini G, Materassi F, Chellini F, Antonelli A, Tani A, Zecchi-Orlandini S, Rossolini GM, Bani D (2016) The effects of diode laser on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide adherent to titanium oxide surface of dental implants. An in vitro study Lasers Med Sci. doi:10.1007/s10103-016-2025-5
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43:109–142
Masini E, Nistri S, Vannacci A, Bani Sacchi T, Novelli A, Bani D (2004) Relaxin inhibits the activation of human neutrophils: involvement of the nitric oxide pathway. Endocrinology 145:1106–1112
Leonhardt A, Bergström C, Lekholm U (2003) Microbiologic diagnostics at titanium implants. Clin Implant Dent Relat Res 5:226–232
Pereira CA, Romeiro RL, Costa ACBP, Machado AKS, Junqueira JC, Jorge AOC (2011) Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: an in vitro study. Lasers Med Sci 26:341–348
Rosa LP, da Silva FC, Nader SA, Meira GA, Viana MS (2015) Antimicrobial photodynamic inactivation of Staphylococcus aureus biofilms in bone specimens using methylene blue, toluidine blue ortho and malachite green: an in vitro study. Arch Oral Biol 60:675–680
Mombelli A (2002) Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000(28):177–189
Usacheva MN, Teichert MC, Biel MA (2003) The interaction of lipopolysaccharides with phenothiazine dyes. Lasers Surg Med 33:311–319
Acknowledgements
The authors are grateful to Dr. Peter Schüpbach, from Schüpbach Ltd. (Thalwil, Switzerland), for conducting the SEM investigation and Dr. Waldemar Hoffmann, Nobel Biocare Services AG (Kloten, Switzerland), for reviewing the manuscript. Moreover, Nobel Biocare Services AG (Kloten, Switzerland) is acknowledged for supporting this study (Grant 2014–1250) and for the generous gift of the titanium discs needed for the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest concerning the contents of this study and that Nobel Biocare Services AG did not influence in any way the analysis or interpretation of the experimental data.
Rights and permissions
About this article
Cite this article
Giannelli, M., Landini, G., Materassi, F. et al. Effects of photodynamic laser and violet-blue led irradiation on Staphylococcus aureus biofilm and Escherichia coli lipopolysaccharide attached to moderately rough titanium surface: in vitro study. Lasers Med Sci 32, 857–864 (2017). https://doi.org/10.1007/s10103-017-2185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2185-y