Abstract
Backgrounds
The presence of ≥5 circulating tumor cells (CTCs) in 7.5 ml blood is a poor prognostic marker in metastatic breast cancer (MBC). However, the role of human epidermal growth factor receptor 2 (HER2) status in CTCs is not known.
Methods
We prospectively assessed the prognostic value of this parameter for patients with MBC who started a new line of systemic therapy. The CTC count (≥5 or <5) and the HER2 status in CTCs at the initiation of the therapy and 3–4 weeks later (first follow-up) were determined.
Results
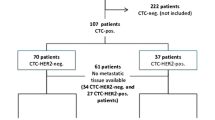
The median follow-up time of the 52 enrolled patients was 655.0 days (18–1,275 days). HER2-positive CTCs were present in 14 of the 52 patients (26.9%) during the study period. Eight of 33 patients (24.2%) with HER2-negative primary tumors had HER2-positive CTCs during the study period. At first follow-up, patients with HER2-positive CTCs had significantly shorter progression-free (n = 6; P = 0.001) and overall (P = 0.013) survival than did patients without HER2-positive CTCs (n = 43) in log-rank analysis. In multivariate analysis, HER2-positive CTCs at first follow-up (P = 0.029) and the number of therapies patients received before this study (P = 0.006) were independent prognostic factors in terms of progression-free survival. The number of therapies (P = 0.001) and a count of ≥5 CTCs (P = 0.043) at baseline were independent prognostic factors in terms of overall survival.
Conclusions
We showed that HER2 status in CTCs may be a prognostic factor for MBC. Well-powered prospective studies are necessary to determine the potential role of HER2-targeted therapies for patients with HER2-positive CTCs and HER2-negative primary tumors.

Similar content being viewed by others
References
Ministry of Health Law: Vital statistics of Japan; 2008. Available from http://www.mhlw.go.jp/english/database/db-hw/vs01.html
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Bartsch R, Wenzel C, Pluschnig U et al (2006) Prognostic value of monitoring tumour markers CA 15-3 and CEA during fulvestrant treatment. BMC Cancer 6:81
Cheung KL, Graves CR, Robertson JF (2000) Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev 26:91–102
Duffy MJ (2005) Serum tumor markers in breast cancer: are they of clinical value? Clin Chem 52:345–351
Khatcheressian JL, Wolff AC, Smith TJ et al (2006) American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol 24:5091–5097
Kurebayashi J, Nishimura R, Tanaka K et al (2004) Significance of serum tumor markers in monitoring advanced breast cancer patients treated with systemic therapy: a prospective study. Breast Cancer 11:389–395
Tampellini M, Berruti A, Bitossi R et al (2006) Prognostic significance of changes in CA 15-3 serum levels during chemotherapy in metastatic breast cancer patients. Breast Cancer Res Treat 98:241–248
Tondini C, Hayes DF, Gelman R et al (1988) Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res 48:4107–4112
Cristofanilli M, Broglio KR, Guarneri V et al (2007) Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 7:471–479
Cristofanilli M, Budd GT, Ellis MJ et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781–791
Cristofanilli M, Hayes DF, Budd GT et al (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23:1420–1430
Hayes DF, Cristofanilli M, Budd GT et al (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12:4218–4224
Nakamura S, Yagata H, Ohno S et al (2010) Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer 17:199–204
Yagata H, Nakamura S, Toi M et al (2008) Evaluation of circulating tumor cells in patients with breast cancer: multi-institutional clinical trial in Japan. Int J Clin Oncol 13:252–256
Apostolaki S, Perraki M, Pallis A et al (2007) Circulating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevance. Ann Oncol 18:851–858
Fehm T, Becker S, Duerr-Stoerzer S et al (2007) Determination of HER2 status using both serum HER2 levels and circulating tumor cells in patients with recurrent breast cancer whose primary tumor was HER2 negative or of unknown HER2 status. Breast Cancer Res 9:R74
Meng S, Tripathy D, Shete S et al (2004) HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci USA 101:9393–9398
Wulfing P, Borchard J, Buerger H et al (2006) HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res 12:1715–1720
Pestrin M, Bessi S, Galardi F et al (2009) Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 118:523–530
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Shimada M, Imura J, Kozaki T et al (2005) Detection of Her2/neu, c-MYC and ZNF217 gene amplification during breast cancer progression using fluorescence in situ hybridization. Oncol Rep 13:633–641
Apostolaki S, Perraki M, Kallergi G et al (2009) Detection of occult HER2 mRNA-positive tumor cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic relevance. Breast Cancer Res Treat 117:525–534
Fehm T, Hoffmann O, Aktas B et al (2009) Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res 11:R59
Ignatiadis M, Kallergi G, Ntoulia M et al (2008) Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res 14:2593–2600
Riethdorf S, Muller V, Zhang L et al (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the Neoadjuvant GeparQuattro Trial. Clin Cancer Res 16:2634–2645
Tewes M, Aktas B, Welt A et al (2009) Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat 115:581–590
Acknowledgments
The authors thank Sachiko Ohde for statistical assistance; Bibari Nakamura, Keiko Shimizu, and all the staff from the Department of Breast Surgical Oncology, St. Luke’s International Hospital, for help in collecting clinical data; Masayuki Shimada, Takeshi Watanabe, and Yuki Matsuo from SRL Inc. for tissue analysis; and Sunita Patterson, Department of Scientific Publications, MD Anderson Cancer Center, for editorial review. This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant, CA016672.
Conflict of interest
Yuji Shimoda is employed by SRL Inc., and SRL Inc. provided analysis of serum HER2 level in the blood samples at St. Luke’s International Hospital (Hayashi N, Nakamura S, Yoshida A, and Yagata H). All other coauthors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hayashi, N., Nakamura, S., Tokuda, Y. et al. Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol 17, 96–104 (2012). https://doi.org/10.1007/s10147-011-0260-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0260-0