Abstract
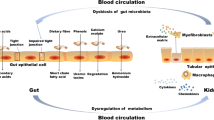
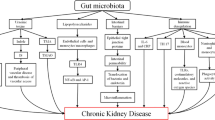
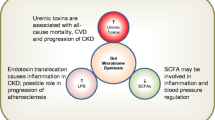
It is well-established that uremic toxins are positively correlated with the risk of developing chronic kidney disease and cardiovascular disease. In addition, emerging data suggest that gut bacteria exert an influence over both the production of uremic toxins and the development of chronic kidney disease. As such, modifying the gut microbiota may have the potential as a treatment for chronic kidney disease. This is supported by data that suggest that rescuing microbiota dysbiosis may: reduce uremic toxin production; prevent toxins and pathogens from crossing the intestinal barrier; and, reduce gastrointestinal tract transit time allowing nutrients to reach the microbiota in the distal portion of the gastrointestinal tract. Despite emerging literature, the gut–kidney axis has yet to be fully explored. A special focus should be placed on examining clinically translatable strategies that might encourage improvements to the microbiome, thereby potentially reducing the risk of the development of chronic kidney disease. This review aims to present an overview of literature linking changes to the gastrointestinal tract with microbiota dysbiosis and the development and progression of chronic kidney disease.

Similar content being viewed by others
Abbreviations
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular disease
- GIT:
-
Gastrointestinal tract
- PCS:
-
p-Cresyl sulfate
- IS:
-
Indoxyl sulfate
- CHO:
-
Carbohydrate
References
Bergstrom A, Skov TH, Bahl MI, Roager HM, Christensen LB, Ejlerskov KT, et al. Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl Environ Microbiol. 2014;80(9):2889–900. doi:10.1128/AEM.00342-14.
Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–45S.
Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859–904. doi:10.1152/physrev.00045.2009.
Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–69. doi:10.1038/nri2710.
Benson AK, Kelly SA, Legge R, Ma F, Low SJ, Kim J, et al. Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc Natl Acad Sci USA. 2010;107(44):18933–8. doi:10.1073/pnas.1007028107.
Phillips ML. Gut reaction: environmental effects on the human microbiota. Environ Health Perspect. 2009;117(5):A198–205.
Scott KP, Gratz SW, Sheridan PO, Flint HJ, Duncan SH. The influence of diet on the gut microbiota. Pharmacol Res: Off J Italian Pharmacol Soc. 2013;69(1):52–60. doi:10.1016/j.phrs.2012.10.020.
Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48. doi:10.1016/j.cell.2006.02.017.
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65.
Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3(6):637–82. doi:10.3390/nu3060637.
Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415):242–9. doi:10.1038/Nature11552.
Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The long-term stability of the human gut microbiota. Science. 2013;341(6141):1237439. doi:10.1126/science.1237439.
Proctor LM. The Human Microbiome Project in 2011 and beyond. Cell Host Microbe. 2011;10(4):287–91. doi:10.1016/j.chom.2011.10.001.
Langhendries JP. Early bacterial colonisation of the intestine: why it matters? Arch Pediatr: Organe Officiel de la Societe Francaise de Pediatrie. 2006;13(12):1526–34. doi:10.1016/j.arcped.2006.09.018.
Turovskiy Y, Sutyak Noll K, Chikindas ML. The aetiology of bacterial vaginosis. J Appl Microbiol. 2011;110(5):1105–28. doi:10.1111/j.1365-2672.2011.04977.x.
Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–7. doi:10.1038/nature09646.
Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7(12):691–701. doi:10.1038/nrgastro.2010.172.
Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–73. doi:10.1128/CMR.00046-08 (Table of contents).
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104(34):13780–5. doi:10.1073/pnas.0706625104.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi:10.1038/nature07540.
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi:10.1371/journal.pone.0009085.
Evenepoel P, Meijers BK, Bammens BR, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl. 2009;114:S12–9. doi:10.1038/ki.2009.402.
Montiel-Castro AJ, Gonzalez-Cervantes RM, Bravo-Ruiseco G, Pacheco-Lopez G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. doi:10.3389/fnint.2013.00070.
Giannelli V, Di Gregorio V, Iebba V, Giusto M, Schippa S, Merli M, et al. Microbiota and the gut-liver axis: bacterial translocation, inflammation and infection in cirrhosis. World J Gastroenterol: WJG. 2014;20(45):16795–810. doi:10.3748/wjg.v20.i45.16795.
Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, et al. Gut–microbiota–metabolite axis in early renal function decline. PLoS One. 2015;10(8):e0134311. doi:10.1371/journal.pone.0134311.
Wang H, Liu JS, Peng SH, Deng XY, Zhu DM, Javidiparsijani S, et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J Gastroenterol: WJG. 2013;19(40):6794–804. doi:10.3748/wjg.v19.i40.6794.
Barrios C, Spector TD, Menni C. Blood, urine and faecal metabolite profiles in the study of adult renal disease. Arch Biochem Biophys. 2015;. doi:10.1016/j.abb.2015.10.006.
Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Kottgen A, Levey AS, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158–69. doi:10.1016/S0140-6736(13)60439-0.
Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80(12):1258–70. doi:10.1038/ki.2011.368.
Johnson DWFK, Harvie B, Jardine M, Katz I, Langham R, Ludlow M, Mathew T, Nelson C, Phoon R, Polkinghorne K, Saunders J, Usherwood T, Wilmott S. Chronic kidney disease management in general practice. 3rd ed. Adelaide: Kidney Health Australia; 2015.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52. doi:10.1016/S0140-6736(13)60595-4.
Chen ZY, Guo LL, Zhang YQ, Walzem RL, Pendergast JS, Printz RL, et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest. 2014;124(8):3391–406. doi:10.1172/Jci72517.
Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–53. doi:10.1007/s10620-011-1887-4.
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi:10.1371/journal.pone.0009085.
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013;83(2):308–15. doi:10.1038/ki.2012.345.
Cummings JH. Fermentation in the human large intestine: evidence and implications for health. Lancet. 1983;1(8335):1206–9.
Poesen R, Viaene L, Verbeke K, Augustijns P, Bammens B, Claes K, et al. Cardiovascular disease relates to intestinal uptake of p-cresol in patients with chronic kidney disease. BMC Nephrol. 2014;15:87. doi:10.1186/1471-2369-15-87.
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. doi:10.1161/CIRCRESAHA.116.305360.
Mishima E, Fukuda S, Shima H, Hirayama A, Akiyama Y, Takeuchi Y, et al. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol: JASN. 2014;. doi:10.1681/ASN.2014060530.
Rossi M, Johnson DW, Xu H, Carrero JJ, Pascoe E, French C, et al. Dietary protein-fiber ratio associates with circulating levels of indoxyl sulfate and p-cresyl sulfate in chronic kidney disease patients. Nut Metab Cardiovasc Dis: NMCD. 2015;. doi:10.1016/j.numecd.2015.03.015.
Yasuda G, Shibata K, Takizawa T, Ikeda Y, Tokita Y, Umemura S, et al. Prevalence of constipation in continuous ambulatory peritoneal dialysis patients and comparison with hemodialysis patients. Am J Kidney Dis: Off J Nat Kidney Found. 2002;39(6):1292–9. doi:10.1053/ajkd.2002.33407.
Wu MJ, Chang CS, Cheng CH, Chen CH, Lee WC, Hsu YH, et al. Colonic transit time in long-term dialysis patients. Am J Kidney Dis: Off J Nat Kidney Found. 2004;44(2):322–7.
Stephen AM, Wiggins HS, Cummings JH. Effect of changing transit time on colonic microbial metabolism in man. Gut. 1987;28(5):601–9.
Resmini E, Parodi A, Savarino V, Greco A, Rebora A, Minuto F, et al. Evidence of prolonged orocecal transit time and small intestinal bacterial overgrowth in acromegalic patients. J Clin Endocrinol Metab. 2007;92(6):2119–24. doi:10.1210/jc.2006-2509.
Macfarlane GT, Macfarlane S. Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J Clin Gastroenterol. 2011;45(Suppl):S120–7. doi:10.1097/MCG.0b013e31822fecfe.
Booijink CC, Zoetendal EG, Kleerebezem M, de Vos WM. Microbial communities in the human small intestine: coupling diversity to metagenomics. Future Microbiol. 2007;2(3):285–95. doi:10.2217/17460913.2.3.285.
Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48(2):206–11.
Roza AM, Edmiston CE Jr, Frantzides C, Moore GH, Nowak TV, Johnson CP, et al. Untreated diabetes mellitus promotes intestinal microbial overgrowth. Am J Surg. 1992;163(4):417–21.
Strid H, Simren M, Stotzer PO, Abrahamsson H, Bjornsson ES. Delay in gastric emptying in patients with chronic renal failure. Scand J Gastroenterol. 2004;39(6):516–20. doi:10.1080/00365520410004505.
Strid H, Simren M, Stotzer PO, Ringstrom G, Abrahamsson H, Bjornsson ES. Patients with chronic renal failure have abnormal small intestinal motility and a high prevalence of small intestinal bacterial overgrowth. Digestion. 2003;67(3):129–37.
Kolida S, Gibson GR. Prebiotic capacity of inulin-type fructans. J Nutr. 2007;137(11 Suppl):2503S–6S.
Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–12.
Cummings JH, Hill MJ, Bone ES, Branch WJ, Jenkins DJ. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr. 1979;32(10):2094–101.
Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. doi:10.2337/db07-1403.
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–55. doi:10.1161/CIRCRESAHA.116.305360.
McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open. 2014;4(8):e005047. doi:10.1136/bmjopen-2014-005047.
Lamprecht M, Bogner S, Schippinger G, Steinbauer K, Fankhauser F, Hallstroem S, et al. Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr. 2012;9(1):45. doi:10.1186/1550-2783-9-45.
Gupta P, Andrew H, Kirschner BS, Guandalini S. Is Lactobacillus GG helpful in children with Crohn’s disease? Results of a preliminary, open-label study. J Pediatr Gastr Nutr. 2000;31(4):453–7. doi:10.1097/00005176-200010000-00024.
Parassol N, Freitas M, Thoreux K, Dalmasso G, Bourdet-Sicard R, Rampal P. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res Microbiol. 2005;156(2):256–62. doi:10.1016/j.resmic.2004.09.013.
Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCzeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol. 2007;9(3):804–16. doi:10.1111/j.1462-5822.2006.00836.x.
Sui W, Tan J, Guo J, Du G, Dai Y. An altered TH1/TH2 and pro-inflammatory cytokine profile in patients with end-stage renal disease detected by suspension array technology. Ren Fail. 2009;31(1):1–5. doi:10.1080/08860220802516449.
Alvarez-Lara MA, Carracedo J, Ramirez R, Martin-Malo A, Rodriguez M, Madueno JA, et al. The imbalance in the ratio of Th1 and Th2 helper lymphocytes in uraemia is mediated by an increased apoptosis of Th1 subset. Nephrol Dial Transplant: Off Publ Eur Dial Transplant Assoc Eur Renal Assoc. 2004;19(12):3084–90. doi:10.1093/ndt/gfh382.
Hwang YJ, Yun MO, Jeong KT, Park JH. Uremic toxin indoxyl 3-sulfate regulates the differentiation of Th2 but not of Th1 cells to lessen allergic asthma. Toxicol Lett. 2014;225(1):130–8. doi:10.1016/j.toxlet.2013.11.027.
Hwang SJ, Hwang YJ, Yun MO, Kim JH, Oh GS, Park JH. Indoxyl 3-sulfate stimulates Th17 differentiation enhancing phosphorylation of c-Src and STAT3 to worsen experimental autoimmune encephalomyelitis. Toxicol Lett. 2013;220(2):109–17. doi:10.1016/j.toxlet.2013.04.016.
Wilson CB. Immunologic basis for increased susceptibility of the neonate to infection. J Pediatr. 1986;108(1):1–12.
Holt PG. Environmental factors and primary T-cell sensitisation to inhalant allergens in infancy: reappraisal of the role of infections and air pollution. Pediatr Allergy Immunol: Off Publ Eur Soc Pediatr Allergy Immunol. 1995;6(1):1–10.
Bowman LM, Holt PG. Selective enhancement of systemic Th1 immunity in immunologically immature rats with an orally administered bacterial extract. Infect Immun. 2001;69(6):3719–27. doi:10.1128/IAI.69.6.3719-3727.2001.
Feng T, Wang L, Schoeb TR, Elson CO, Cong Y. Microbiota innate stimulation is a prerequisite for T cell spontaneous proliferation and induction of experimental colitis. J Exp Med. 2010;207(6):1321–32. doi:10.1084/jem.20092253.
Crabb JH, Finberg R, Onderdonk AB, Kasper DL. T cell regulation of Bacteroides fragilis-induced intraabdominal abscesses. Rev Infect Dis. 1990;12(Suppl 2):S178–84.
Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–98. doi:10.1016/j.cell.2009.09.033.
Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, et al. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA. 1996;93(21):11774–9.
Dalton JE, Cruickshank SM, Egan CE, Mears R, Newton DJ, Andrew EM, et al. Intraepithelial gammadelta + lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131(3):818–29. doi:10.1053/j.gastro.2006.06.003.
Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182(5):3047–54. doi:10.4049/jimmunol.0802705.
Simenhoff ML, Dunn SR, Zollner GP, Fitzpatrick ME, Emery SM, Sandine WE, et al. Biomodulation of the toxic and nutritional effects of small bowel bacterial overgrowth in end-stage kidney disease using freeze-dried Lactobacillus acidophilus. Miner Electrolyte Metab. 1996;22(1–3):92–6.
Ranganathan N, Patel BG, Ranganathan P, Marczely J, Dheer R, Pechenyak B, et al. In vitro and in vivo assessment of intraintestinal bacteriotherapy in chronic kidney disease. ASAIO J. 2006;52(1):70–9. doi:10.1097/01.mat.0000191345.45735.00.
Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25(8):1919–30. doi:10.1185/03007990903069249.
Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther. 2010;27(9):634–47. doi:10.1007/s12325-010-0059-9.
Guida B, Germano R, Trio R, Russo D, Memoli B, Grumetto L, et al. Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis: NMCD. 2014;24(9):1043–9. doi:10.1016/j.numecd.2014.04.007.
Cruz-Mora J, Martinez-Hernandez NE, Martin del Campo-Lopez F, Viramontes-Horner D, Vizmanos-Lamotte B, Munoz-Valle JF, et al. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr: Off J Counc Ren Nutr Nat Kidney Found. 2014;24(5):330–5. doi:10.1053/j.jrn.2014.05.006.
Miranda Alatriste PV, Urbina Arronte R, Gomez Espinosa CO, Espinosa Cuevas Mde L. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp. 2014;29(3):582–90. doi:10.3305/nh.2014.29.3.7179.
Kajander K, Myllyluoma E, Rajilic-Stojanovic M, Kyronpalo S, Rasmussen M, Jarvenpaa S, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27(1):48–57. doi:10.1111/j.1365-2036.2007.03542.x.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. doi:10.1038/nature12820.
Buffie CG, Jarchum I, Equinda M, Lipuma L, Gobourne A, Viale A, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80(1):62–73. doi:10.1128/IAI.05496-11.
Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi:10.1371/journal.pbio.0060280.
Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1(1):56–66. doi:10.1038/ismej.2007.3.
Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, et al. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature. 2013;502(7469):96–9. doi:10.1038/nature12503.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
About this article
Cite this article
Briskey, D., Tucker, P., Johnson, D.W. et al. The role of the gastrointestinal tract and microbiota on uremic toxins and chronic kidney disease development. Clin Exp Nephrol 21, 7–15 (2017). https://doi.org/10.1007/s10157-016-1255-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-016-1255-y