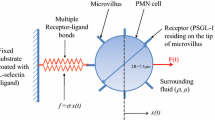
Abstract
L-selectin–PSGL-1-mediated polymorphonuclear (PMN) leukocyte homotypic interactions potentiate the extent of PMN recruitment to endothelial sites of inflammation. Cell–cell adhesion is a complex phenomenon involving the interplay of bond kinetics and hydrodynamics. As a first step, a 3-D computational model based on the Immersed Boundary Method is developed to simulate adhesion-detachment of two PMN cells in quiescent conditions. Our simulations predict that the total number of bonds formed is dictated by the number of available receptors (PSGL-1) when ligands (L-selectin) are in excess, while the excess amount of ligands influences the rate of bond formation. Increasing equilibrium bond length results in a higher number of receptor–ligand bonds due to an increased intercellular contact area. On-rate constants determine the rate of bond formation, while off-rates control the average number of bonds by modulating bond lifetimes. Application of an external pulling force leads to time-dependent on- and off-rates and causes bond rupture. Moreover, the time required for bond rupture in response to an external force is inversely proportional to the applied load and decreases with increasing off-rate.
Similar content being viewed by others
References
Alon R, Hammer DA, Springer TA (1995) Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature 374: 539–542
Alon R, Fuhlbrigge RC, Finger ER, Springer TA (1996) Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol 135(3): 849–865
Bargatze RF, Kurk S, Butcher EC, Jutila MA (1994) Neutrophils roll on adherent netrophils bond to cytokine-induced endothelial cells via L-selectin on rolling cells. J Exp Med 180: 1785–1792
Bell GI (1978) Models for the specific adhesion of cells to cells. Science 200: 618–627
Bruehl RE, Springer TA, Bainton DF (1996) Quantification of L-selectin distribution on human leukocyte microvilli by immunogold labeling and electron microscopy. J Histochem Cytochem 44(8): 835–844
Charrier JM, Shrivastava S, Wu R (1989) Free and constrained inflation of elastic membranes in relation to thermoforming non-axisymmetric problems. J Strain Anal 24(2): 55–74
Chen S, Springer TA (1999) An automatic braking system that stabilizes leukocyte rolling by an increase in selectin bond number with shear. J Cell Biol 144: 185–200
Chen W, Zarnitsyna VI, Sarangapani KK, Huang J, Zhu C (2008) Measuring receptor-ligand binding kinetics on cell surfaces: from adhesion frequency to thermal fluctuation methods. Cell Mol Bioeng 1(4): 276–288
Chesla SE, Selvaraj P, Zhu C (1998) Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophys J 75: 1553–1572
Cozen-Roberts C, Lauffenburger DA, Quinn JA (1990) Receptor- mediated cell attachment and detachment kinetics. I. Probabilistic model and analysis. Biophys J 58: 841–856
Dembo M (1994) On peeling an adherent cell from a surface. In: vol 24 of series: Lectures on Mathematics in the Life Sciences, Some Mathematical Problems in Biology. American Mathematical Society, Providence, RI. pp 51–77
Eggleton CD, Popel AS (1998) Large deformation of red blood cell ghosts in a simple shear flow. Phys Fluids 10: 1834–1845
Evans E, Ritchie K (1997) Dynamic strength of molecular adhesion bonds. Biophys J 72: 1541–1555
Evans EA, Leung A, Hammer D, Simon S (2001) Chemically distinct transition states govern rapid dissociation of single L-selectin bonds under force. Proc Natl Acad Sci 98(7): 3784–3789
Evans EA, Calderwood DA (2007) Forces and bond dynamics in cell adhesion. Science 316: 1148–1153
Fritz J, Katopodis AG, Kolbinger F, Anselmetti D (1998) Force- mediated kinetics of single P-selectin/ligand complexes observed by atomic force microscopy. Proc Natl Acad Sci 95: 12283–12288
Girdhar G, Shao J-Y (2007) Simultaneous tether extraction from endothelial cells and leukocytes: observation, mechanics, and significance. Biophys J 93: 4041–4052
Goldsmith HL, Quinn TA, Drury G, Spanos C, McIntosh FA, Simon SI (2001) Dynamics of neutrophil aggregation in Couette flow revealed by videomicroscopy: effect of shear rate on two-body collision efficiency and doublet lifetime. Biophys J 81: 2020– 2034
Guo S, Ray C, Kirkpatrick A, Lad N, Akhremitchev BB (2008) Effects of multiple-bond ruptures on kinetic parameters extracted from force spectroscopy measurements: revisiting biotin-streptavidin interactions. Biophys J 95: 3964–3976
Guo S, Lad N, Ray C, Akhremitchev BB (2009) Association kinetics from single molecule force spectroscopy measurements. Biophys J 96: 3412–3422
Hammer DA, Apte SM (1992) Simulation of cell rolling and adhesion on surfaces in shear flow: general results and analysis of selectin-mediated neutrophil adhesion. Biophys J 63: 35–57
Hinterdorfer P, Baumgartner W, Gruber HJ, Schilcher K, Schindler H (1996) Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. PNAS 93: 3477–3481
Jadhav S, Eggleton CD, Konstantopoulos K (2005) A 3-D computational model predicts that cell deformation affects selectin-mediated leukocyte rolling. Biophy J 88: 96–104
Jadhav S, Chan KY, Eggleton CD, Konstantopoulos K (2007) Shear modulation of intercellular contact area between two deformable cells colliding under flow. J Biomech 40: 2891–2897
Kadash KE, Lawrence MB, Diamond SL (2004) Neutrophil string formation: hydrodynamic thresholding and cellular deformation during cell collisions. Biophys J 86: 4030–4039
Khismatullin DB, Truskey GA (2005) Three-dimensional numerical simulation of receptor-mediated leukocyte adhesion to surfaces: effects of cell deformability and viscoelasticity. Phys Fluids 17: 031505
Konstantopoulos K, Kukreti S, McIntire LV (1998) Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev 33: 141–164
Kuhner F, Costa LT, Bisch PM, Thalhammer S, Heckl WM, Gaub HE (2004) LexA-DNA bond strength by single molecule force spectroscopy. Biophys J 87: 2683–2690
Lasky LA (1992) Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science 258: 964–969
Lawrence MB, Springer TA (1991) Leukocytes roll on a selectin at physiologic flow rates: distinction from the prerequisite for adhesion through integrins. Cell 65: 859–873
Marshall BT, Sarangapani KK, Wu J, Lawrence MB, McEver RP (2006) Measuring molecular elasticity by atomic force microscope cantilever fluctuations. Biophys J 90: 681–692
Merkel R, Nassoy P, Leung A, Ritchie K, Evans E (1999) Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature 397: 50–53
Moore KL, Varki A, McEver RP (1991) GMP-140 binds to a glycoprotein receptor on human neutrophils: evidence for a lectin-like interaction. J Cell Biol 112: 491–499
Moore KL, Patel KD, Bruehl RE, Li F, Johnson DA, Lichenste HS, Cummings RD, Bainton DF, McEver RP (1995) P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol 128: 661–671
Mustard JF, Packham MA, Kinlough-Rathbone RL, Perry DW, Regoeczi E (1978) Fibrinogen and ADP-induced platelet aggregation. Blood 52: 453–466
Neelamegham S, Taylor AD, Shankaran H, Smith CW, Simon SI (2000) Shear and time-dependent changes in Mac-1, LFA-1, and ICAM-3 binding regulate neutrophil homotypic adhesion. J Immunol 164: 3798–3805
Nicolson GL (1988) Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev 7: 143–188
Osborn L (1990) Leukocyte adhesion to endothelium in inflammation. Cell 62: 3–6
Paschall CD, Guilford WH, Lawrence MB (2008) Enhancement of L-selectin, but not P-selectin, bond formation frequency by convective flow. Biophys J 94: 1034–1045
Patel KD, Nollert MU, McEver RP (1995) P-selectin must extend a sufficient length from the plasma membrane to mediate rolling of neutrophils. J Cell Biol 131(6): 1893–1902
Pawar P, Jadhav S, Eggleton CD, Konstantopoulos K (2008) Roles of cell and microvillus deformation and receptor-ligand binding kinetics in cell rolling. Am J Physiol Heart Circ Physiol 295: H1439–H1450
Pereverzev YV, Prezhdo OV, Forero M, Sokurenko EV, Thomas WE (2005) The two-pathway model for the catch-slip transition in biological adhesion. Biophys J 89: 1446–1454
Peskin CS, McQueen DM (1989) A three dimensional computational method for blood flow in the heart. I. Immersed elastic fibers in a viscous incompressible fluid. J Comput Phys 81: 372
Puri KD, Finger EB, Springer TA. (1997) The faster kinetics of L-selectin than of E-selectin and P-selectin rolling at comparable binding strength. J Immunol 158: 405–413
Rinko LJ, Lawrence MB, Guilford WH (2004) The molecular mechanics pf P- and L-selectin lectin domains binding to PSGL-1. Biophys J 86: 544–554
Shao JY, Xu JB (2002) A modified micropipette aspiration technique and its application to tether formation from human neutrophils. J Biomech Eng-T ASME 124: 388–396
Shao JY, Ting-Beall HP, Hochmuth RM (1998) Static and dynamic lengths of neutrophil microvilli. Proc Natl Acad Sci USA 95: 6797–6802
Shrivastava S, Tang J (1993) Large deformation finite element analysis of non-linear viscoelastic membranes with reference to thermoforming. J Strain Anal 28(1): 31–51
Simon SI, Green CE (2005) Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7: 151–185
Tandon P, Diamond SL (1998) Kinetics of β 2-integrin and L-selectin bonding during neutrophile aggregation in shear flow. Biophys J 75: 3163–3178
Taylor AD, Neelamegham S, Hellums JD, Smith CW, Simon SI (1996) Molecular dynamics of the transition from L-selectin to β 2-integrin dependent neutrophil adhesion under defined hydrodynamic shear. Biophys J 71: 3488–3500
Tolentino TP, Wu J, Zarnitsyna VI, Fang Y, Dustin ML, Zhu C (2008) Measuring diffusion and binding kinetics by contact area FRAP. Biophys J 95: 920–930
Williams PM (2003) Analytical descriptions of dynamic force spectroscopy: behaviour of multiple connections. Anal Chim Acta 479: 107–115
Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C (2007) Transport governs flow-enhanced cell tethering through L-selectin at threhold shear. Biophys J 92: 330–342
Zhang X, Bogorin DF, Moy VT (2004) Molecular basis of the dynamic strength of the sialyl Lewis X-selectin interaction. Chemphyschem 5: 175–182
Zhao Y, Chien S, Weinbaum S (2001) Dynamic contact forces on leukocyte microvilli and their penetration of the endothelial glycocalyx. Biophys J 80: 1124–1140
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, V.K., Sraj, I.A., Konstantopoulos, K. et al. Multi-scale simulation of L-selectin–PSGL-1-dependent homotypic leukocyte binding and rupture. Biomech Model Mechanobiol 9, 613–627 (2010). https://doi.org/10.1007/s10237-010-0201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0201-2