Abstract
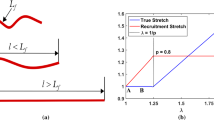
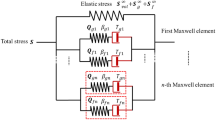
In this paper, we studied the viscoelastic behaviors of isolated aortic elastin using combined modeling and experimental approaches. Biaxial stress relaxation and creep experiments were performed to study the time-dependent behavior of elastin. Experimental results reveal that stress relaxation preconditioning is necessary in order to obtain repeatable stress relaxation responses. Elastin exhibits less stress relaxation than intact or decellularized aorta. The rate of stress relaxation of intact and decellularized aorta is linearly dependent on the initial stress levels. The rate of stress relaxation for elastin increases linearly at stress levels below about 60 kPa; however, the rate changes very slightly at higher initial stress levels. Experimental results also show that creep response is negligible for elastin, and the intact or decellularized aorta. A quasi-linear viscoelasticity model was incorporated into a statistical mechanics based eight-chain microstructural model at the fiber level to simulate the orthotropic viscoelastic behavior of elastin. A user material subroutine was developed for finite element analysis. Results demonstrate that this model is suitable to capture both the orthotropic hyperelasticity and viscoelasticity of elastin.
Similar content being viewed by others
References
Agabiti-Rosei E, Portei E, Rizzoni D (2009) Arterial stiffness, hypertension, and rational use of nebivolol. Vasc Health Risk Manag 5: 353–360
Atkinson TS, Ewers BJ, Haut RC (1999) The tensile and stress relaxation responses of human patellar tendon varies with specimen cross-sectional area. J Biomec 32: 907–914
Bader A, Schilling T, Teebken OE, Brandes G, Herden T, Steinhoff G, Haverich A (1998) Tissue engineering of heart valves-human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg 14: 279–284
Bergstrom JS, Boyce MC (2001) Constitutive modeling of the time-dependent and cyclic loading of elastomers and application to soft biological tissues. Mech Mater 33: 523–530
Best TM, McElhaney J, Garret WE, Myers BS (1994) Characterization of the passive responses of live skeletal muscle using the quasilinear theory of viscoelasticity. J Biomec 27: 413–419
Bischoff JE, Arruda EM, Grosh K (2002) Orthotropic hyperelasticity in terms of an arbitrary molecular chain model. J Appl Mech 69: 198–201
Bischoff JE, Arruda EM, Grosh K (2004) A rheological network model for the continuum anisotropic and viscoelastic behavior of soft tissue. Biomech Model Mechanobiol 3: 56–65
Bischoff JE (2006) Reduced parameter formulation for incorporating fiber level viscoelasticity into tissue level biomechanical models. Ann Biomed Eng 34: 1164–1172
Boutouyrie P, Laurent S, Briet M (2008) Importance of arterial stiffness as cardiovascular risk factor for future development of new type of drugs. Fundam Clin Pharmacol 22: 241–246
Boyce BL, Jones RE, Nguyen TD, Grazier JM (2007) Stress-controlled viscoelastic tensile response of bovine cornea. J Biomec 40: 2367–2376
Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C (2003) The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care 26: 2133–2140
Campa JS, Greenhalgh RM, Powell JT (1987) Elastin degradation in abdominal aortic aneurysms. Atherosclerosis 65: 13–21
Carew EO, Talman EA, Boughnew DR, Vesely I (1999) Quasi-linear viscoelastic theory applied to internal shearing of porcine aortic valve leaflets. J Biomech Eng 121: 386–392
Carew EO, Barber JE, Vesely I (2000) Role of preconditioning and recovery time in repeated testing of aortic valve tissues: validation through quasi-linear viscoelastic theory. Ann Biomed Eng 28: 1093–1100
Carew EO, Garg A, Barber JE, Vesely I (2004) Stress relaxation preconditioning of porcine aortic valves. Ann Biomed Eng 32: 563–572
Chung AW, Yang HH, Sigrist MK, Brin G, Chum E, Gourlay WA, Levin A (2009) Matrix metalloproteinase-2 and −9 exacerbate arterial stiffening and angiogenesis in diabetes and chronic kidney disease. Cardiovasc Res. doi:10.1093/cvr/cvp242
Cox RH (1978) Passive mechanics and connective tissue composition of canine arteries. Am J Physiol Heart Circ Physiol 234: H533–H541
Daamen WF, Veerkamp JH, van Hest JCM, van Kuppevelt TH (2007) Elastin as a biomaterial for tissue engineering. Biomaterials 28: 4378–4398
Diez J (2007) Arterial stiffness and extracellular matrix. Adv Cardiol 44: 76–95
Doehring TC, Carew EO, Vesely I (2004) The effect of strain rate on the viscoelastic response of aortic valve tissue: a direct-fit approach. Ann Biomed Eng 32: 223–232
Fonck E, Prod’hom G, Roy S, Augsburger L, Rufenacht DA, Stergiopulos N (2007) Effect of elastin degradation on carotid wall mechanics as assessed by a constituent-based biomechanical model. Am J Physiol Heart Circ Physiol 292: H2754–H2763
Fung YC (1993) Biomechanics: mechanical properties of living tissues. Springer, New York
Geest JPV, Sacks MS, Vorp DA (2006) The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J Biomec 39: 1324–1334
Giles JM, Black AE, Bischoff JE (2007) Anomalous rate dependence of the preconditioned response of soft tissue during load controlled deformation. J Biomec 40: 777–785
Gosline JM (1976) The physical properties of elastic tissue. Int Rev Connect Tissue Res 7: 211–249
Grashow JS, Sacks MS, Liao J, Yoganathan AP (2006) Planar biaxial creep and stress relaxation of the mitral valve anterior leaflet. Ann Biomed Eng 34: 1509–1518
Guinea GV, Atienza JM, Elices M, Aragoncillo P, Hayashi K (2005) Thermomechanical behavior of human carotid arteries in the passive state. Am J Physiol Heart Circ Physiol 288: H2940–H2945
Gundiah N, Ratcliffe MB, Pruitt LA (2009) The biomechanics of arterial elastin. J Mech Behav Biomed Mater 2: 288–296
Gundiah N, Ratcliffe MB, Pruitt LA (2007) Determination of strain energy function for arterial elastin: Experiments using histology and mechanical tests. J Biomec 40: 586–594
Guo X, Kassab GS (2004) Distribution of stress and strain along the porcine aorta and coronary arterial tree. Am J Physiol Heart Circ Physiol 286: H2361–H2368
Hingorani RV, Provenzano PP, Lakes RS, Escarcega A, Vanderby R Jr (2004) Nonlinear viscoelasticity in rabbit medial collateral ligament. Ann Biomed Eng 32: 306–312
Holzapfel GA (2000) Nonlinear solid mechanics: a continuum approach for engineering. Wiley, Chichester
Holzapfel GA, Gasser TC, Stadler M (2002) A structural model for the viscoelastic behavior of arterial walls: continuum formulation and finite element analysis. Eur J Mech A Solids 21: 441–463
Huang CY, Wang VM, Flatow EL, Mow VC (2009) Temperature dependent viscoelastic properties of the human supraspinatus tendon. J Biomec 42: 546–549
Humphrey JD (2002) Cardiovascular solid mechanics: cells, tissues, and organs. Springer, New York
Jhun CS, Evans MC, Barocas VH, Tranquillo RT (2009) Planar biaxial mechanical behavior of bioartificial tissues possessing prescribed fiber alignment. J Biomech Eng 131: 081006
Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL (1994) Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12: 796–803
Kang T, Resar J, Humphrey JD (1995) Heat-induced changes in the mechanical behavior of passive coronary arteries. J Biomech Eng 117: 86–93
Knauss WG, Emri IJ (1981) Non-linear viscoelasticity based on free volume consideration. Comput Struct 13: 123–128
Knezevic V, Sim AJ, Borg TK, Holmes JW (2002) Isotonic biaxial loading of fibroblast-populated collagen gels: a versatile, low-cost system for the study of mechanobiology. Biomech Model Mechanobiol 1: 59–67
Kwan MK, Li THD, Woo SLY (1993) On the viscoelastic properties of the anteromedial bundle of the anterior cruciate ligament. J Biomec 26: 47–452
Lally C, Reid AJ, Prendergast PJ (2004) Elastic behavior of porcine coronary artery tissue under uniaxial and equibiaxial tension. Ann Biomed Eng 32: 1355–1364
Liao J, Vesely I (2004) Relationship between collagen fibrils, glycosaminoglycans, and stress relaxation in mitral valve chordae tendineae. Ann Biomed Eng 32: 977–983
Liao J, Yang L, Grashow J, Sacks MS (2007) The relationship between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng 129: 78–87
Lillie MA, Gosline JM (1990) The effects of hydration on the dynamic mechanical properties of elastin. Biopolymers 29: 1147–1160
Lillie MA, Gosline JM (1996) Swelling and viscoelastic properties of osmotically stressed elastin. Biopolymers 39: 641–693
Lillie MA, Gosline JM (2002) The viscoelastic basis for the tensile strength of elastin. Int J Biol Macromol 30: 119–127
Lillie MA, Gosline JM (2007) Limits to the durability of arterial elastic tissue. Biomaterials 28: 2021–2031
Lu Q, Ganesan K, Simionescu DT, Vyavahare NR (2004) Novel porous aortic elastin and collagen scaffolds for tissue engineering. Biomaterials 25: 5227–5237
Lyerla JR Jr, Torchia DA (1975) Molecular mobility and structure of elastin deduced from the solvent and temperature dependence of 13C magnetic resonance relaxation data. Biochemistry 14: 5175–5183
MacSweeney STR, Young G, Greenhalgh RM, Powell JT (1992) Mechanical properties of the aneurismal aorta. Br J Surg 79: 1281–1284
MacSweeney STR, Powell JT, Greenhalgh RM (1994) Pathogenesis of abdominal aortic aneurysms. Br J Surg 81: 935–941
Mow VC, Kuei SC, Lai WM, Armstrong CG (1980) Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng 102: 73–84
Nagatomi J, Toosi KK, Chancellor MB, Sacks MS (2008) Contribution of the extracellular matrix to the viscoelastic behavior of the urinary bladder wall. Biomech Model Mechanobiol 7: 395–404
Nagatomi J, Gloeckner DC, Chancellor MB, DeGroat WC, Sacks MS (2004) Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Ann Biomed Eng 32: 1409–1419
Nguyen TD, Jones RE, Boyce BL (2008) A nonlinear anisotropic viscoelastic model for the tensile behavior of the corneal stroma. J Biomech Eng 130: 041020
Öhman C, Baleani M, Viceconti M (2009) Repeatability of experimental procedures to determine mechanical behavior of ligaments. Acta Bioeng Biomech 11: 19–23
Provenzano P, Lakes R, Keenan T, Vanderby R Jr (2001) Nonlinear ligament viscoelasticity. Ann Biomed Eng 29: 908–914
Puso MA, Weiss JA (1998) Finite element implementation of anisotropic quasi-linear viscoelasticity using a discrete spectrum approximation. J Biomech Eng 120: 62–70
Sacks MS, Sun W (2003) Multiaxial mechanical behavior of biological materials. Ann Rev Biomed Eng 5: 251–284
Satta J, Laurila A, Pääkkö P, Haukipuro K, Sormunen R, Parkkila S, Juvonen T (1998) Chronic inflammation and elastin degradation in abdominal aortic aneurysm disease: an immunohistochemical and electron microscopic study. Eur J Vasc Endovasc Surg 15: 313–319
Sauren AAHJ, van Hout MC, Steenhoven AA, Veldpaus FE, Janssen JD (1983) The mechanical properteies of porcine aortic valve tissues. J Biomec 16: 327–337
Silver FH, Horvath I, Foran DJ (2001) Viscoelasticity of the vessel wall: the role of collagen and elastic fibers. Crit Rev Biomed Eng 29: 279–301
Soulhat J, Buschmann MD, Shirazi-Adl A (1999) A fibril-network-reinforced biphasic model of cartilage in unconfined compression. J Biomech Eng 121: 340–347
Spina M, Friso A, Ewins AR, Parker KH, Winlove CP (1999) Physicochemical properties of arterial elastin and its associated glycoproteins. Biopolymers 49: 255–265
Stella JA, Liao J, Sacks MS (2007) Time-dependent biaxial mechanical behavior of the aortic heart valve leaflet. J Biomec 40: 3169–3177
Stephens EH, Chu CK, Grande-Allen KJ (2008) Valve proteoglycan content and glycosaminoglycan fine structure are unique to microstructure, mechanical load and age: relevance to an age-specific tissue-engineered heart valve. Acta Biomaterialia 4: 1148–1160
Stergiopulos N, Vulliemoz S, Rachev A, Meister J-J, Greenwald SE (2001) Assessing the homogeneity of the elastic properties and composition of the pig aortic media. J Vasc Res 38: 237–246
Sverdlik A, Lanir Y (2002) Time-dependent mechanical behavior of sheep digital tendons, including the effects of preconditioning. J Biomech Eng 124: 78–84
Thornton GM, Oliynyk A, Frank CB, Shrive NG (1997) Ligament creep cannot be predicted from stress relaxation at low stress: a biomechanical study of the rabbit medial collateral ligament. J Orthop Res 15: 652–656
Thornton GM, Frank CB, Shrive NG (2001) Ligament creep behavior can be predicted from stress relaxation by incorporating fiber recruitment. J Rheol 45: 493–507
Uitto J (1979) Biochemistry of the elastic fibers in normal connective tissues and its alterations in diseases. J Invest Dermatol 72: 1–10
von Maltzahn WW, Warriyar RG (1984) Experimental measurement of elastic properties of media and adventitia of bovine carotid arteries. J Biomech 17: 839–847
Weinberg PD, Winlove CP, Parker KH (1995) The distribution of water in arterial elastin: effects of mechanical stress, osmotic pressure, and temperature. Biopolymers 35: 161–169
Williams C, Liao J, Joyce EM, Wang B, Leach JB, Sacks MS, Wong JY (2009) Altered structural and mechanical properties in decellularized rabbit carotid arteries. Acta Biomaterialia 5: 993–1005
Winlove CP, Parker KH (1990) Connective tissue matrix II. In: Hukins DWL (ed) MacMillan, London, chap 7
Yin FCP, Chew PH, Zeger SL (1986) An approach to quantification of biaxial tissue stress-strain data. J Biomec 19: 27–37
Zhang Y, Dunn ML, Drexler ES, McCowan CN, Slifka AJ, Ivy DD, Shandas R (2005) A microstructural hyperelastic model of pulmonary arteries under normo- and hypertensive conditions. Ann Biomed Eng 33: 1042–1052
Zou Y, Zhang Y (2009) An experimental and theoretical study on the anisotropy of elastin network. Ann Biomed Eng 37: 1572–1583
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zou, Y., Zhang, Y. The orthotropic viscoelastic behavior of aortic elastin. Biomech Model Mechanobiol 10, 613–625 (2011). https://doi.org/10.1007/s10237-010-0260-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-010-0260-4