Abstract
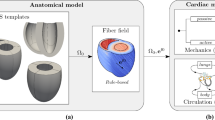
Modeling of the heart ventricles is one of the most challenging tasks in soft tissue mechanics because cardiac tissue is a strongly anisotropic incompressible material with an active component of stress. In most current approaches with active force, the number of degrees of freedom (DOF) is limited by the direct method of solution of linear systems of equations. We develop a new approach for high-resolution heart models with large numbers of DOF by: (1) developing a hex-dominant finite element mixed formulation and (2) developing a Krylov subspace iterative method that is able to solve the system of linearized equations for saddle-point problems with active stress. In our approach, passive cardiac tissue is modeled as a hyperelastic, incompressible material with orthotropic properties, and mixed pressure-displacement finite elements are used to enforce incompressibility. Active stress is generated by a model with force dependence on length and velocity of muscle shortening. The ventricles are coupled to a lumped circulatory model. For efficient solution of linear systems, we use Flexible GMRES with a nonlinear preconditioner based on block matrix decomposition involving the Schur complement. Three methods for approximating the inverse of the Schur complement are evaluated: inverse of the pressure mass matrix; least squares commutators; and sparse approximate inverse. The sub-matrix corresponding to the displacement variables is preconditioned by a V-cycle of hybrid geometric–algebraic multigrid followed by correction with several iterations of GMRES preconditioned by sparse approximate inverse. The overall solver is demonstrated on a high-resolution two ventricle mesh based on a human anatomy with roughly 130 K elements and 1.7 M displacement DOF. Effectiveness of the numerical method for active contraction is shown. To the best of our knowledge, this solver is the first to efficiently model ventricular contraction using an iterative linear solver for the mesh size demonstrated and therefore opens the possibility for future very high-resolution models. In addition, several relatively simple benchmark problems are designed for a verification exercise to show that the solver is functioning properly and correctly solves the underlying mathematical model. Here, the output of the newly designed solver is compared to that of the mechanics component of Chaste (‘Cancer, Heart and Soft Tissue Environment’). These benchmark tests may be used by other researchers to verify their newly developed methods and codes.










Similar content being viewed by others
Notes
We are using the \(l^2\) norm. Residual entries have weights 1 kPa\(^{-1}\)mm\(^{-2}\) and 1 mm\(^{-3}\) for the displacement and pressure equations, respectively. For the volume constraints, the residual weight was \(\frac{1}{V_\mathrm{u}}\), where \(V_\mathrm{u}\) is the unloaded volume of the ventricle.
Hypre implements right preconditioning for GMRES.
For instance, for edge nodes \(n_i=2\), for brick face nodes \(n_i=4\), for the interior node of the brick element \(n_i=8\).
See Chaste documentation, e.g., https://chaste.cs.ox.ac.uk/trac/wiki/ChasteGuides/FiniteElementImplementations.
Tetrahedral meshes were generated using the mesh generator Tetgen http://wias-berlin.de/software/tetgen/.
SuperLU was used to invert the sparse matrix \(A_{\mathrm{pc}}\), and LAPACK was used to invert the dense matrix \(S_{\mathrm{pc}}\).
References
Ackerman MJ (1998) The visible human project. Proc IEEE 86(3):504–511
Augustin CM, Holzapfel GA, Steinbach O (2014) Classical and all-floating FETI methods for the simulation of arterial tissues. Int J Numer Methods Eng 99(4):290–312
Auricchio F, da Veiga LB, Lovadina C, Reali A, Taylor RL, Wriggers P (2013) Approximation of incompressible large deformation elastic problems: some unresolved issues. Comput Mech 52(5):1153–1167
Baker A, Kolev TV, Yang U (2010) Improving algebraic multigrid interpolation operators for linear elasticity problems. Numer Linear Algebra Appl 17(2–3):495–517
Bröker O, Grote MJ, Mayer C, Reusken A (2001) Robust parallel smoothing for multigrid via sparse approximate inverses. SIAM J Sci Comput 23(4):1396–1417
Campbell SG, Howard E, Aguado-Sierra J, Coppola BA, Omens JH, Mulligan LJ, McCulloch AD, Kerckhoffs RC (2009) Effect of transmurally heterogeneous myocyte excitation–contraction coupling on canine left ventricular electromechanics. Exp Physiol 94(5):541–552
Chamberland É, Fortin A, Fortin M (2010) Comparison of the performance of some finite element discretizations for large deformation elasticity problems. Comput Struct 88(11):664–673
Cgal, Computational Geometry Algorithms Library. http://www.cgal.org
Chapelle D, Bathe K (1993) The inf-sup test. Comput Struct 47(4):537–545
Costa KD, Holmes JW, McCulloch AD (2001) Modelling cardiac mechanical properties in three dimensions. Philos Trans R Soc Lond Ser A 359(1783):1233–1250
Council NR (2012) Assessing the reliability of complex models: mathematical and statistical foundations of verification, validation, and uncertainty quantification. The National Academies Press. http://www.nap.edu/openbook.php?record_id=13395
El maliki A, Fortin M, Tardieu N, Fortin A (2010) Iterative solvers for 3D linear and nonlinear elasticity problems: displacement and mixed formulations. Int J Numer Methods Eng 83(13):1780–1802
El maliki A, Guenette R, Fortin M (2011) An efficient hierarchical preconditioner for quadratic discretizations of finite element problems. Numer Linear Algebra Appl 18(5):789–803
Elman H, Howle V, Shadid J, Shuttleworth R, Tuminaro R (2006) Block preconditioners based on approximate commutators. SIAM J Sci Comput 27(5):1651–1668
Farhat C, Roux FX (1991) A method of finite element tearing and interconnecting and its parallel solution algorithm. Int J Numer Methods Eng 32(6):1205–1227
Fortin M, Glowinski R (2000) Augmented Lagrangian methods: applications to the numerical solution of boundary-value problems. Elsevier, Amsterdam
Fritz T, Wieners C, Seemann G, Steen H, Dössel O (2013) Simulation of the contraction of the ventricles in a human heart model including atria and pericardium. Biomech Model Mechanobiol 13(3):627–641
Göktepe S, Acharya S, Wong J, Kuhl E (2011) Computational modeling of passive myocardium. Int J Numer Methods Biomed Eng 27(1):1–12
Golub GH, Greif C (2003) On solving block-structured indefinite linear systems. SIAM J Sci Comput 24(6):2076–2092
Guccione J, McCulloch A, Waldman L et al (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng 113(1):42
Gurev V, Lee T, Constantino J, Arevalo H, Trayanova NA (2011) Models of cardiac electromechanics based on individual hearts imaging data. Biomech Model Mechanobiol 10(3):295–306
Heil M, Hazel AL (2006) Fluid-structure interaction. Lecture notes in computational science and engineering, vol 53, pp. 19–49
Holzapfel GA, Ogden RW (2009) Constitutive modelling of passive myocardium: a structurally based framework for material characterization. Philos Trans R Soc A 367(1902):3445–3475
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
Hypre. High performance preconditioners. http://www.llnl.gov/CASC/linear_solvers
Jie X, Gurev V, Trayanova N (2010) Mechanisms of mechanically induced spontaneous arrhythmias in acute regional ischemia. Circ Res 106(1):185–192
Keldermann RH, Nash MP, Gelderblom H, Wang VY, Panfilov AV (2010) Electromechanical wavebreak in a model of the human left ventricle. Am J Physiol Cell Physiol 299(1):H134
Kerckhoffs R, Lumens J, Vernooy K, Omens J, Mulligan L, Delhaas T, Arts T, McCulloch A, Prinzen F (2008) Cardiac resynchronization: insight from experimental and computational models. Prog Biophys Mol Biol 97(2):543–561
Kerckhoffs RC, Neal ML, Gu Q, Bassingthwaighte JB, Omens JH, McCulloch AD (2007) Coupling of a 3D finite element model of cardiac ventricular mechanics to lumped systems models of the systemic and pulmonic circulation. Ann Biomed Eng 35(1):1–18
Klepach D, Lee LC, Wenk JF, Ratcliffe MB, Zohdi TI, Navia JL, Kassab GS, Kuhl E, Guccione JM (2012) Growth and remodeling of the left ventricle: a case study of myocardial infarction and surgical ventricular restoration. Mech Res Commun 42:134–141
Land S, Niederer SA, Aronsen JM, Espe EK, Zhang L, Louch WE, Sjaastad I, Sejersted OM, Smith NP (2012) An analysis of deformation-dependent electromechanical coupling in the mouse heart. J Physiol 590(18):4553–4569
Le Tallec P (1994) Numerical methods for nonlinear three-dimensional elasticity. In: Ciarlet P, lions J (eds) Handbook of numerical analysis, vol III. Elsevier Science, Amsterdam, p 465
Lumens J, Delhaas T, Kirn B, Arts T (2009) Three-wall segment (triseg) model describing mechanics and hemodynamics of ventricular interaction. Ann Biomed Eng 37(11):2234–2255
Mirams G, Arthurs C, Bernabeu M, Bordas R, Cooper J, Corrias A, Davit Y, Dunn SJ, Fletcher A, Harvey D, Marsh M, Osborne J, Pathmanathan P, Pitt-Francis J, Southern J, Zemzemi N, Gavaghan D (2013). Chaste: an open source C++ library for computational physiology and biology. PloS Comput Biol 9(3):e1002970
Murphy MF, Golub GH, Wathen AJ (2000) A note on preconditioning for indefinite linear systems. SIAM J Sci Comput 21(6):1969–1972
Niederer S, Kerfoot E, Benson A, Bernabeu M, Bernus O, Bradley C, Cherry E, Clayton R, Fenton F, Garny A et al (2011) Verification of cardiac tissue electrophysiology simulators using an n-version benchmark. Philos Trans R Soc A 369(1954):4331–4351
Niederer SA, Smith NP (2008) An improved numerical method for strong coupling of excitation and contraction models in the heart. Prog Biophys Mol Biol 96(1–3):90–111
Nobile F, Quarteroni A, Ruiz-Baier R (2012) An active strain electromechanical model for cardiac tissue. Int J Numer Methods Biomed Eng 28(1):52–71
Notay Y (2013) A new analysis of block preconditioners for saddle point problems. SIAM J Matrix Anal Appl 35(1):143–173
Oberkampf W, Trucano T, Hirsch C (2004) Verification, validation, and predictive capability in computational engineering and physics. Appl Mech Rev 57(5):345–384
Pantuso D, Bathe KJ (1997) On the stability of mixed finite elements in large strain analysis of incompressible solids. Finite Elem Anal Des 28(2):83–104
Pathmanathan P, Chapman S, Gavaghan D, Whiteley J (2010) Cardiac electromechanics: the effect of contraction model on the mathematical problem and accuracy of the numerical scheme. Q J Mech Appl Math 63(3):375–399
Pathmanathan P, Gray RA (2013) Ensuring reliability of safety-critical clinical applications of computational cardiac models. Front Physiol 4:358
Pathmanathan P, Gray R (2014). Verification of computational models of cardiac electrophysiology. Int J Numer Methods Biomed Eng 30(5):525–544
Pitt-Francis J, Pathmanathan P, Bernabeu M, Bordas R, Cooper J, Fletcher A, Mirams G, Murray P, Osbourne J, Walter A, Chapman S, Garny A, van Leeuwen I, Maini P, Rodriguez B, Waters S, Whiteley J, Byrne H, Gavaghan D (2009) Chaste: a test-driven approach to software development for biological modelling. Comput Phys Commun 180:2452–2471
Prassl AJ, Kickinger F, Ahammer H, Grau V, Schneider JE, Hofer E, Vigmond EJ, Trayanova NA, Plank G (2009) Automatically generated, anatomically accurate meshes for cardiac electrophysiology problems. IEEE Trans Biomed Eng 56(5):1318–1330
Razumova MV, Bukatina AE, Campbell KB (1999) Stiffness-distortion sarcomere model for muscle simulation. J Appl Physiol 87(5):1861–1876
Richards DF, Glosli JN, Draeger EW, Mirin AA, Chan B, Fattebert JL, Krauss WD, Oppelstrup T, Butler CJ, Gunnels JA et al (2013) Towards real-time simulation of cardiac electrophysiology in a human heart at high resolution. Comput Methods Biomech Biomed Eng 16(7):802–805
Roache P (1998) Verification and validation in computational science and engineering. Hermosa, Albuquerque
Rossi S, Lassila T, Ruiz-Baier R, Sequeira A, Quarteroni A (2013). Thermodynamically consistent orthotropic activation model capturing ventricular systolic wall thickening in cardiac electromechanics. Eur J Mech A Solids 48:129–142
Saad Y (1993) A flexible inner–outer preconditioned GMRES algorithm. SIAM J Sci Comput 14(2):461–469
Saad Y, Schultz MH (1986) GMRES: a generalized minimal residual algorithm for solving nonsymmetric linear systems. SIAM J Sci Comput 7:856–869
Schroder JB (2012) Smoothed aggregation solvers for anisotropic diffusion. Numer Linear Algebra Appl 19:296–312
Usyk T, Mazhari R, McCulloch A (2000) Effect of laminar orthotropic myofiber architecture on regional stress and strain in the canine left ventricle. J Elast Phys Sci Solids 61(1–3):143–164
Usyk TP, LeGrice IJ, McCulloch AD (2002) Computational model of three-dimensional cardiac electromechanics. Comput Vis Sci 4(4):249–257
Wang H, Gao H, Luo X, Berry C, Griffith B, Ogden R, Wang T (2013) Structure-based finite strain modelling of the human left ventricle in diastole. Int J Numer Methods Biomed Eng 29(1):83–103
Wang H, Luo X, Gao H, Ogden R, Griffith B, Berry C, Wang T (2014) A modified Holzapfel-Ogden law for a residually stressed finite strain model of the human left ventricle in diastole. Biomech Model Mechanobiol 13(1):99–113
Washio T, Hisada T (2011) Convergence analysis of inexact LU-type preconditioners for indefinite problems arising in incompressible continuum analysis. Jpn J Ind Appl Math 28(1):89–117
Washio T, Okada Ji, Takahashi A, Yoneda K, Kadooka Y, Sugiura S, Hisada T (2013) Multiscale heart simulation with cooperative stochastic cross-bridge dynamics and cellular structures. Multiscale Model Simul 11(4):965–999
Weiss J, Maker B, Govindjee S (1996) Finite element implementation of incompressible, transversely isotropic hyperelasticity. Comput Methods Applied Mech Eng 135(1):107–128
Acknowledgments
We thank Tzanio Kolev and Ulrike Meier Yang from the Hypre team at Lawrence Livermore National Laboratory for very informative discussions. The work of J.-L. Fattebert and D. F. Richards was performed under the auspices of the US Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
Conflict of interest
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Gurev and P. Pathmanathan contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gurev, V., Pathmanathan, P., Fattebert, JL. et al. A high-resolution computational model of the deforming human heart. Biomech Model Mechanobiol 14, 829–849 (2015). https://doi.org/10.1007/s10237-014-0639-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-014-0639-8