Abstract
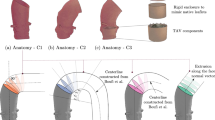
Credible computational fluid dynamic (CFD) simulations of aortic dissection are challenging, because the defining parallel flow channels—the true and the false lumen—are separated from each other by a more or less mobile dissection membrane, which is made up of a delaminated portion of the elastic aortic wall. We present a comprehensive numerical framework for CFD simulations of aortic dissection, which captures the complex interplay between physiologic deformation, flow, pressures, and time-averaged wall shear stress (TAWSS) in a patient-specific model. Our numerical model includes (1) two-way fluid–structure interaction (FSI) to describe the dynamic deformation of the vessel wall and dissection flap; (2) prestress and (3) external tissue support of the structural domain to avoid unphysiologic dilation of the aortic wall and stretching of the dissection flap; (4) tethering of the aorta by intercostal and lumbar arteries to restrict translatory motion of the aorta; and a (5) independently defined elastic modulus for the dissection flap and the outer vessel wall to account for their different material properties. The patient-specific aortic geometry is derived from computed tomography angiography (CTA). Three-dimensional phase contrast magnetic resonance imaging (4D flow MRI) and the patient’s blood pressure are used to inform physiologically realistic, patient-specific boundary conditions. Our simulations closely capture the cyclical deformation of the dissection membrane, with flow simulations in good agreement with 4D flow MRI. We demonstrate that decreasing flap stiffness from \({E}_{\hbox{flap}}= 800\) to \({E}_{\hbox{flap}}= 20\) kPa (a) increases the displacement of the dissection flap from 1.4 to 13.4 mm, (b) decreases the surface area of TAWSS by a factor of 2.3, (c) decreases the mean pressure difference between true lumen and false lumen by a factor of 0.63, and (d) decreases the true lumen flow rate by up to 20% in the abdominal aorta. We conclude that the mobility of the dissection flap substantially influences local hemodynamics and therefore needs to be accounted for in patient-specific simulations of aortic dissection. Further research to accurately measure flap stiffness and its local variations could help advance future CFD applications.












Similar content being viewed by others
References
Alimohammadi M, Sherwood JM, Karimpour M, Agu O, Balabani S, Díaz-Zuccarini V (2015) Aortic dissection simulation models for clinical support: fluid-structure interaction versus rigid wall models. Biomed Eng online 14(1):34
Bazilevs Y, Calo VM, Cottrell JA, Hughes TJR, Reali A, Scovazzi G (2007) Variational multiscale residual-based turbulence modeling for large eddy simulation of incompressible flows. Comput Methods Appl Mech Eng 197(1–4):173–201
Bazilevs Y, Calo VM, Hughes TJR, Zhang Y (2008) Isogeometric fluid-structure interaction: theory, algorithms, and computations. Comput Mech 43(1):3–37
Bazilevs Y, Hsu M-Y, Zhang Y, Wang W, Kvamsdal T, Hentschel S, Isaksen JG (2010) Computational vascular fluid-structure interaction: methodology and application to cerebral aneurysms. Biomech Model Mechanobiol 9(4):481–498
Berguer R, Parodi JC, Schlicht M, Khanafer K (2015) Experimental and clinical evidence supporting septectomy in the primary treatment of acute type B thoracic aortic dissection. Ann Vasc Surg 29(2):167–173
Bonfanti M, Balabani S, Greenwood JP, Puppala S, Homer-Vanniasinkam S, Díaz-Zuccarini V (2017) Computational tools for clinical support: a multi-scale compliant model for haemodynamic simulations in an aortic dissection based on multi-modal imaging data. J R Soc Interface 14(136):20170632
Bonfanti M, Balabani S, Alimohammadi M, Agu O, Homer-Vanniasinkam S, Díaz-Zuccarini V (2018) A simplified method to account for wall motion in patient-specific blood flow simulations of aortic dissection: comparison with fluid-structure interaction. Med Eng Phys 58:72–79
Canchi S, Guo X, Phillips M, Berwick Z, Kratzberg J, Krieger J, Roeder B, Haulon S, Chambers S, Kassab GS (2017) Role of re-entry tears on the dynamics of type B dissection flap. Ann Biomed Eng 46:186–196
Chen HY, Peelukhana SV, Berwick ZC, Kratzberg J, Krieger JF, Roeder B, Chambers S, Kassab GS (2016) Editor’s choice—fluid-structure interaction simulations of aortic dissection with bench validation. Eur J Vasc Endovasc Surg 52(5):589–595
Cheng Z, Tan FPP, Riga CV, Bicknell CD, Hamady MS, Gibbs RGJ, Wood NB, Xu XY (2010) Analysis of flow patterns in a patient-specific aortic dissection model. Journal of biomechanical engineering 132(5):51007
Cheng Z, Juli C, Wood NB, Gibbs RGJ, Xu XY (2014a) Predicting flow in aortic dissection: comparison of computational model with PC-MRI velocity measurements. Med Eng Phys 36(9):1176–1184
Cheng Z, Wood NB, Gibbs RGJ, Xu XY (2014b) Geometric and flow features of type B aortic dissection: initial findings and comparison of medically treated and stented cases. Ann Biomed Eng 43(1):177–189
Chiu P, Miller DC (2016) Evolution of surgical therapy for Stanford acute type A aortic dissection. Asvide 3(4):313. 10.21037/asvide.2016.313
Chung JW, Elkins C, Sakai T, Kato N, Vestring T, Semba CP, Slonim SM, Dake MD (2000a) True-lumen collapse in aortic dissection: part I. Evaluation of causative factors in phantoms with pulsatile flow. Radiology 214:87–98
Chung JW, Elkins C, Sakai T, Kato N, Vestring T, Semba CP, Slonim SM, Dake MD (2000b) True-lumen collapse in aortic dissection part II. Evaluation of treatment. Radiology 214:99–106
Di Achille P, Tellides G, Humphrey JD (2017) Hemodynamics-driven deposition of intraluminal thrombus in abdominal aortic aneurysms. Int J Numer Methods Biomed Eng 33(5):1–17
Dillon-Murphy D, Noorani A, Nordsletten D, Figueroa CA (2016) Multi-modality image-based computational analysis of haemodynamics in aortic dissection. Biomech Model Mechanobiol 15(4):857–876
Doyle BJ, Norman PE (2016) Computational biomechanics in thoracic aortic dissection: today’s approaches and tomorrow’s opportunities. Annals of Biomedical Engineering 44(1):71–83
Esmaily-Moghadam M, Bazilevs Y, Marsden AL (2013) A new preconditioning technique for implicitly coupled multidomain simulations with applications to hemodynamics. Comput Mech 52(5):1141–1152
Esmaily Moghadam M, Vignon-Clementel IE, Figliola R, Marsden AL (2013) A modular numerical method for implicit 0D/3D coupling in cardiovascular finite element simulations. J Comput Phys 244:63–79
Esmaily-Moghadam M, Bazilevs Y, Marsden AL (2015) A bi-partitioned iterative algorithm for solving linear systems arising from incompressible flow problems. Comput Methods Appl Mech Eng 286:40–62
Ganten M, Weber TF, Von Tengg-Kobligk H, Böckler D, Stiller W, Geisbüsch P, Kauffmann GW, Delorme S, Bock M, Kauczor H (2009) Motion characterization of aortic wall and intimal flap by ECG-gated CT in patients with chronic B-dissection. Eur J Radiol 72:146–153
Greve JM, Les AS, Tang BT, Draney Blomme MT, Wilson NM, Dalman RL, Pelc NJ, Taylor CA (2006) Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol 291(4):H1700–H1708. 10.1152/ajpheart.00274.2006
Holzapfel G (2000) Nonlinear solid mechanics: a continuum approach for engineering
Hsiao A, Yousaf U, Alley MT, Lustig M, Chan FP, Newman B, Vasanawala SS (2015) Improved quantification and mapping of anomalous pulmonary venous flow with four-dimensional phase-contrast MRI and interactive streamline rendering. J Magn Reson Imaging 42(6):1765–1776
Hsu M-C, Bazilevs Y (2011) Blood vessel tissue prestress modeling for vascular fluid–structure interaction simulation. Finite Elem Anal Des 47(6):593–599
Karmonik C, Bismuth J, Shah DJ, Davies MG, Purdy D, Lumsden AB (2011) Computational study of haemodynamic effects of entry- and exit-tear coverage in a DeBakey type III aortic dissection: technical report. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg 42(2):172–177
Kassab GS (2006) Biomechanics of the cardiovascular system: the aorta as an illustratory example. J R Soc Interface/R Soc 3(11):719–40
Kim HJ, Vignon-Clementel IE, Figueroa Ca, Jansen KE, Taylor Ca (2010) Developing computational methods for three-dimensional finite element simulations of coronary blood flow. Finite Elem Anal Des 46(6):514–525 ISSN 0168874X
Laskey WK, Parker HG, Ferrari VA, Kussmaul WG, Noordergraaf A (19900) Estimation of total systemic arterial compliance in humans. J Appl Physiol (Bethesda, Md.: 1985), 69(1):112–119, ISSN 8750-7587. http://www.ncbi.nlm.nih.gov/pubmed/2394640
Lesage D, Angelini ED, Bloch I, Funka-Lea G (2009) A review of 3D vessel lumen segmentation techniques: models, features and extraction schemes. Med Image Anal 13(6):819–845
Maher G, Wilson NW, Marsden AL (2019) Accelerating cardiovascular model building with convolutional neural networks. In: Summer biomechanics, bioengineering and biotransport conference
Marsden AL (2013) Simulation based planning of surgical interventions in pediatric cardiology. Phys Fluids 25(10):101303
Martufi G, Forneris A, Appoo JJ, Di Martino ES (2016) Is there a role for biomechanical engineering in helping to elucidate the risk profile of the thoracic aorta? Ann Thorac Surg 101(1):390–398
Menichini C, Cheng Z, Gibbs RGJ, Xu XY (2016) Predicting false lumen thrombosis in patient-specific models of aortic dissection. J R Soc Interface. 10.1098/rsif.2016.0759
Menichini C, Cheng Z, Gibbs RGJ, Xu XY (2018) A computational model for false lumen thrombosis in type B aortic dissection following thoracic endovascular repair. J Biomech 66:36–43
Merkow J, Kriegman D, Marsden A, Zhuowen T (2016) Dense volume-to-volume vascular boundary detection. In: MICCAI
Moireau P, Xiao N, Astorino M, Figueroa CA, Chapelle D, Taylor CA, Gerbeau JF (2012) External tissue support and fluid-structure simulation in blood flows. Biomech Model Mechanobiol 11(1–2):1–18
Moireau P, Bertoglio C, Xiao N, Figueroa CA, Taylor CA, Chapelle D, Gerbeau JF (2013) Sequential identification of boundary support parameters in a fluid-structure vascular model using patient image data. Biomech Model Mechanobiol 12(3):475–496
Nichols WW, O’Rourke MF (2005) McDonald’s blood flow in arteries 5Ed: theoretical, experimental and clinical principles. Taylor & Francis, London
Osswald A, Karmonik C, Anderson JR, Rengier F, Karck M, Engelke J, Kallenbach K, Kotelis D, Partovi S, Böckler D, Ruhparwar A (2017) Elevated wall shear stress in aortic type B dissection may relate to retrograde aortic type a dissection: a computational fluid dynamics pilot study. Eur J Vasc Endovasc Surg 54(3):324–330
Pasta S, Phillippi JA, Gleason TG, Vorp DA (2012) Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg 143(2):460–467
Peelukhana SV, Wang Y, Berwick Z, Kratzberg J, Krieger J, Roeder R, Cloughs RE, Hsiao A, Chambers S, Kassab GS (2017) Role of pulse pressure and geometry of primary entry tear in acute type B dissection propagation. Ann Biomed Eng 45(3):592–603. 10.1007/s10439-016-1705-4
Peterss S, Mansour AM, Ross JA, Vaitkeviciute I, Charilaou P, Dumfarth J, Fang H, Ziganshin BA, Rizzo JA, Adeniran AJ, Elefteriades JA (2016) Changing pathology of the thoracic aorta from acute to chronic dissection: literature review and insights. J Am Coll Cardiol 68(10):1054–1065
Qiao A, Yin W, Chu B (2015) Numerical simulation of fluid-structure interaction in bypassed DeBakey III aortic dissection. Comput Methods Biomech Biomed Eng 18(11):1173–1180
Qiao Y, Zeng Y, Ding Y, Fan J, Luo K, Zhu T (2019) Numerical simulation of two-phase non-Newtonian blood flow with fluid-structure interaction in aortic dissection. Comput Methods Biomech Biomed Eng 22(6):620–630
Reymond P, Crosetto P, Deparis S, Quarteroni A, Stergiopulos N (2013) Physiological simulation of blood flow in the aorta: comparison of hemodynamic indices as predicted by 3-D FSI, 3-D rigid wall and 1-D models. Med Eng Phys 35(6):784–791
Rosero EB, Peshock RM, Khera A, Clagett P, Lo H, Timaran CH (2011) Sex, race, and age distributions of mean aortic wall thickness in a multiethnic population-based sample. J Vasc Surg 53(4):950–957
Sailer AM, van Kuijk SM, Nelemans PJ, Chin AS, Kino A, Huininga M, Schmidt J, Mistelbauer G, Bäumler K, Chiu P, Fischbein MP, Dake MD, Miller DC, Schurink GW, Fleischmann D (2017a) Computed tomography imaging features in acute uncomplicated Stanford type-B aortic dissection predict late adverse events. Circ Cardiovasc Imaging 10(4):e005709
Sailer AM, Nelemans PJ, Hastie TJ, Chin AS, Huininga M, Chiu P, Fischbein MP, Dake MD, Miller DC, Schurink GW, Fleischmann D (2017b) Prognostic significance of early aortic remodeling in acute uncomplicated type B aortic dissection and intramural hematoma. J Thorac Cardiovasc Surg 154(4):1192–1200. https://doi.org/10.1016/j.jtcvs.2017.04.064
Seo J, Schiavazzi DE, Marsden AL (2019) Performance of preconditioned iterative linear solvers for cardiovascular simulations in rigid and deformable vessels. Comput Mech. ISSN 01787675
Shang EK, Nathan DP, Fairman RM, Bavaria JE, Gorman RC, Gorman JH, Jackson BM (2015) Use of computational fluid dynamics studies in predicting aneurysmal degeneration of acute type B aortic dissections. J Vasc Surg 62(2):279–284
Si H (2008) Adaptive tetrahedral mesh generation by constrained Delaunay refinement. Int J Numer Meth Eng 75:856–880
Simo JC, Hughes TJR (1998) Comput Inelasticity
Sommer G, Sherifova S, Oberwalder PJ, Dapunt OE, Ursomanno PA, DeAnda A, Griffith BE, Holzapfel GA (2016) Mechanical strength of aneurysmatic and dissected human thoracic aortas at different shear loading modes. J Biomech 49(12):2374–2382
Spinelli D, Benedetto F, Donato R, Piffaretti G, Marrocco-Trischitta MM, Patel HJ, Eagle KA, Trimarchi S (2018) Current evidence in predictors of aortic growth and events in acute type B aortic dissection. J Vasc Surg 68(6):1925–1935.e8
Takizawa K, Tezduyar TE, Sasaki T (2018) Estimation of element-based zero-stress state in arterial FSI computations with isogeometric wall discretization. Lect Notes Appl Comput Mech 84:101–122
Tang DG, Dake MD (2009) TEVAR for acute uncomplicated aortic dissection: immediate repair versus medical therapy. Semin Vasc Surg 22(3):145–151
Taylor CA, Steinman DA (2010) Image-based modeling of blood flow and vessel wall dynamics: applications, methods and future directions: sixth international bio-fluid mechanics symposium and workshop, March 28–30, 2008 Pasadena, California. Ann Biomed Eng 38(3):1188–1203
Tezduyar TE, Sathe S (2007) Modelling of fluid-structure interaction with the space-time finite elements: solution techniques. Int J Numer Meth Fluids 54(October 2007):855–900
Tezduyar TE, Mittal S, Ray SE, Shih R (1992) Incompressible flow computations with stabilized bilinear and linear equal-order-interpolation velocity-pressure elements. Comput Methods Appl Mech Eng 95(2):221–242
Tezduyar TE, Sathe S, Schwaab M, Conklin BS (2007) Arterial fluid mechanics modeling with the stabilized space-time fluid—structure interaction technique. Int J Numer Methods Fluids 601–629:2008
Towns J, Cockerill T, Dahan M, Foster I, Gaither K, Grimshaw A, Hazlewood V, Lathrop S, Lifka D, Peterson GD, Roskies R, Scott JR, Wilkins-Diehr N (2014) Xsede: accelerating scientific discovery. Comput Sci Eng 16(5):62–74
Trimarchi S, Tolenaar JL, Jonker FHW, Murray B, Tsai TT, Eagle KA, Rampoldi V, Verhagen HJM, Van Herwaarden JA, Moll FL, Muhs BE, Elefteriades JA (2013) Importance of false lumen thrombosis in type B aortic dissection prognosis. J Thorac Cardiovasc Surg 145(3 SUPPL.):S208–S212
Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, Smith DE, Suzuki T, Fattori R, Llovet A, Froehlich J, Hutchison S, Distante A, Sundt T, Beckman J, Januzzi JL, Isselbacher EM, Eagle KA (2007) Partial Thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 357(4):349–359
Tsai TT, Schlicht MS, Khanafer K, Bull JL, Valassis DT, Williams DM, Berguer R, Eagle KA (2008) Tear size and location impacts false lumen pressure in an ex vivo model of chronic type B aortic dissection. Journal of Vascular Surgery 47(4):844–851
Updegrove A, Wilson NM, Merkow J, Lan H, Marsden AL, Shadden SC (2016) SimVascular: an open source pipeline for cardiovascular simulation. Ann Biomed Eng 45(3):525–541. https://doi.org/10.1007/s10439-016-1762-8
Wan Ab Naim WN, Ganesan PB, Sun Z, Chee KH, Hashim SA, Lim E (2014) A perspective review on numerical simulations of hemodynamics in aortic dissection. Sci World J. https://doi.org/10.1155/2014/652520
Yamada H, Sakata N, Wada H, Tashiro T, Tayama E (2015) Age-related distensibility and histology of the ascending aorta in elderly patients with acute aortic dissection. J Biomech 48(12):3267–3273
Yang S, Li X, Chao B, Wu L, Cheng Z (2014) Abdominal aortic intimal flap motion characterization in acute aortic dissection: assessed with retrospective ECG-gated thoracoabdominal aorta dual-source CT angiography. PLoS ONE 9(2):87664
Yu SCH, Liu W, Wong RHL, Underwood M, Wang D (2016) The potential of computational fluid dynamics simulation on serial monitoring of hemodynamic change in type B aortic dissection. Cardiovasc interv Radiol 39:1090–1098
Acknowledgements
This work used the Extreme Science and Engineering Discovery Environment [(XSEDE), Towns et al. (2014)], on cluster Stampede2 at UT Austin, through allocation of P.I. Alison Marsden. XSEDE is supported by National Science Foundation Grant Number ACI-1548562. This work used the Stanford Research Computing Center (SRCC). Additionally, we acknowledge the open-source projects Paraview at www.paraview.org, Meshmixer at www.meshmixer.com and the open-source SimVascular project at www.simvascular.org. This research was funded by the Stanford Cardiovascular Institute (Grant No. N/A).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bäumler, K., Vedula, V., Sailer, A.M. et al. Fluid–structure interaction simulations of patient-specific aortic dissection. Biomech Model Mechanobiol 19, 1607–1628 (2020). https://doi.org/10.1007/s10237-020-01294-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01294-8