Abstract
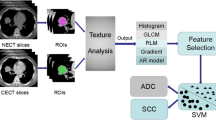
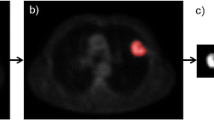
We investigated the association between the textural features obtained from 18F-FDG images, metabolic parameters (SUVmax, SUVmean, MTV, TLG), and tumor histopathological characteristics (stage and Ki-67 proliferation index) in non-small cell lung cancer (NSCLC). The FDG-PET images of 67 patients with NSCLC were evaluated. MATLAB technical computing language was employed in the extraction of 137 features by using first order statistics (FOS), gray-level co-occurrence matrix (GLCM), gray-level run length matrix (GLRLM), and Laws’ texture filters. Textural features and metabolic parameters were statistically analyzed in terms of good discrimination power between tumor stages, and selected features/parameters were used in the automatic classification by k-nearest neighbors (k-NN) and support vector machines (SVM). We showed that one textural feature (gray-level nonuniformity, GLN) obtained using GLRLM approach and nine textural features using Laws’ approach were successful in discriminating all tumor stages, unlike metabolic parameters. There were significant correlations between Ki-67 index and some of the textural features computed using Laws’ method (r = 0.6, p = 0.013). In terms of automatic classification of tumor stage, the accuracy was approximately 84% with k-NN classifier (k = 3) and SVM, using selected five features. Texture analysis of FDG-PET images has a potential to be an objective tool to assess tumor histopathological characteristics. The textural features obtained using Laws’ approach could be useful in the discrimination of tumor stage.




Similar content being viewed by others
References
Abe K, Baba S, Kaneko K, Isoda T, Yabuuchi H, Sasaki M, Sakai S, Yoshino I, Honda H: Diagnostic and prognostic values of FDG-PET in patients with non-small cell lung cancer. Clin Imag 33:90–95, 2009
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, Scherpereel A, Mascaux C, Moreau M, Roelandts M, Alard S, Meert AP, Patz, Jr EF, Lafitte JJ, Sculier JP, European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project: Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol 3:6–12, 2008
Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, Humm JL: Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol 62:545–553, 2005
Weber WA, Schwaiger M, Avril N: Quantitative assessment of tumor metabolism using FDG-PET imaging. Nucl Med Biol 27:683–687, 2000
van Velden FH, Cheebsumon P, Yaqub M, Smit EF, Hoekstra OS, Lammertsma AA, Boellaard R: Evaluation of a cumulative SUV-volume histogram method for parameterizing heterogeneous intratumoural FDG uptake in non-small cell lung cancer PET studies. Eur J Nucl Med Mol Imaging 38:1636–1647, 2011
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ: Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging 40:133–140, 2013
Dong X, Xing L, Wu P, Fu Z, Wan H, Li D, Yin Y, Sun X, Yu J: Three-dimensional positron emission tomography image texture analysis of esophageal squamous cell carcinoma: relationship between tumor 18F-fluorodeoxyglucose uptake heterogeneity, maximum standardized uptake value, and tumor stage. Nucl Med Commun 34:40–46, 2013
Orlhac F, Soussan M, Maisonobe J-A, Garcia CA, Vanderlinden B, Buvat I: Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med 55:414–422, 2014
Bagci U, Yao J, Miller-Jaster K, Chen X, Mollura DJ: Predicting future morphological changes of lesions from radiotracer uptake in 18F-FDG-PET images. PloS One 8(2):e57105, 2013
Cook GJ, Yip C, Siddique M, Goh V, Chicklore S, Roy A, Marsden P, Ahmad S, Landau D: Are pretreatment 18F-FDG PET tumor textural features in non-small cell lung cancer associated with response and survival after chemoradiotherapy? J Nucl Med 54:19–26, 2013
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges J-P, Corcos L, Visvikis D: Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 52:369–378, 2011
Tixier F, Hatt M, Valla C, Fleury V, Lamour C, Ezzouhri S, Ingrand P, Perdrisot R, Visvikis D, Le Rest CC: Visual versus quantitative assessment of intratumor 18F-FDG PET uptake heterogeneity: prognostic value in non–small cell lung cancer. J Nucl Med 55:1235–1241, 2014
Yang F, Thomas MA, Dehdashti F, Grigsby PW: Temporal analysis of intratumoral metabolic heterogeneity characterized by textural features in cervical cancer. Eur J Nucl Med Mol Imaging 40:716–727, 2013
Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D: Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med 53:693–700, 2012
Vaidya M, Creach KM, Frye J, Dehdashti F, Bradley JD, El Naqa I: Combined PET/CT image characteristics for radiotherapy tumor response in lung cancer. Radiother Oncol 102:239–245, 2012
Soussan M, Orlhac F, Boubaya M, Zelek L, Ziol M, Eder V, Buvat I: Relationship between tumor heterogeneity measured on FDG-PET/CT and pathological prognostic factors in invasive breast cancer. PloS One 9(4):e94017, 2014
Ha S, Choi H, Cheon GJ, Kang KW, Chung J-K, Kim EE, Lee DS: Autoclustering of non-small cell lung carcinoma subtypes on 18F-FDG PET using texture analysis: a preliminary result. Nucl Med Mol Imaging 48:278–286, 2014
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A Eds: AJCC cancer staging manual, 7th edition. New York: Springer, 2010
Brooks FJ, Grigsby PW: The effect of small tumor volumes on studies of intratumoral heterogeneity of tracer uptake. J Nuclear Med 55:37–42, 2014
Larson SM, Erdi Y, Akhurst T, Mazumdar M, Macapinlac HA, Finn RD, Casilla C, Fazzari M, Srivastava N, Yeung HW, Humm JL, Guillem J, Downey R, Karpeh M, Cohen AE, Ginsberg R: Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: the visual response score and the change in total lesion glycolysis. Clin Positron Imaging 2:159–171, 1999
Otsu N: A threshold selection method from gray-level histograms. Automatica. 11:23–27, 1975
Sathya P, Kayalvizhi R: Optimal segmentation of brain MRI based on adaptive bacterial foraging algorithm. Neurocomputing 74:2299–2313, 2011
Wang R, Li C, Wang J, Wei X, Li Y, Zhu Y, Zhang S: Threshold segmentation algorithm for automatic extraction of cerebral vessels from brain magnetic resonance angiography images. J Neurosci Methods 241:30–36, 2015
Bashar MK, Komatsu K, Fujimori T, Kobayashi TJ: Automatic extraction of nuclei centroids of mouse embryonic cells from fluorescence microscopy images. PloS one 7(5):e35550, 2012
Bagci U, Foster B, Miller-Jaster K, Luna B, Dey B, Bishai WR, Jonsson CB, Jain S, Mollura DJ: A computational pipeline for quantification of pulmonary infections in small animal models using serial PET-CT imaging. EJNMMI Res 3:55, 2013
Clausi DA: An analysis of co-occurrence texture statistics as a function of grey level quantization. Can J Remote Sens 28:45–62, 2002
Selvarajah S, Kodituwakku S: Analysis and comparison of texture features for content based image retrieval. Int J Latest Trends Computing 2(1):108–113, 2011
Haralick RM, Shanmugam K, Dinstein IH: (1973) Textural features for image classification. IEEE Trans Syst Man Cybern Syst 610–21
Galloway MM: Texture analysis using gray level run lengths. Comp Vision Graph 4:172–179, 1975
Laws KI. Textured image segmentation [Ph.D. thesis] (1980): University of Southern California, Los Angeles
Kayaaltı Ö, Aksebzeci BH, Karahan İÖ, Deniz K, Öztürk M, Yılmaz B, Kara S, Asyalı MH: Liver fibrosis staging using CT image texture analysis and soft computing. Appl Soft Comput 25:399–413, 2014
Chu A, Sehgal C, Greenleaf JF: Use of gray value distribution of run lengths for texture analysis. Pattern Recogn Lett 11:415–419, 1990
Dasarathy BV, Holder EB: Image characterizations based on joint gray level-run length distributions. Pattern Recogn Lett 12:497–502, 1991
Subramanya M, Kumar V, Mukherjee S, Saini M: (2014) A CAD system for B-mode fatty liver ultrasound images using texture features. J Med Eng Technol 1–8
Rachidi M, Marchadier A, Gadois C, Lespessailles E, Chappard C, Benhamou C: Laws’ masks descriptors applied to bone texture analysis: an innovative and discriminant tool in osteoporosis. Skeletal Radiol 37:541–548, 2008
Balagurunathan Y, Kumar V, Gu Y, Kim J, Wang H, Liu Y, Goldgof DB, Hall LO, Korn R, Zhao B, Schwartz LH, Basu S, Eschrich S, Gatenby RA, Gillies RJ: Test–retest reproducibility analysis of lung CT image features. J Digit Imaging 27:805–823, 2014
Tukey JW (1949): Comparing individual means in the analysis of variance. Biometrics 99–114.
Benjamini Y, Hochberg: (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B Met 289–300
Miklavčič D, Pavšelj N, Hart FX: Wiley encyclopedia of biomedical engineering. Malden: John Wiley & Sons, Inc, 2006
Cortes C, Vapnik V: Support-vector networks. Mach Learn 20:273–297, 1995
Pattern PR: Recognition. In: Akay M Ed.. Wiley Encyclopedia of Biomedical Engineering. New York: Wiley, 2006
Whitney AW: A direct method of nonparametric measurement selection. IEEE Trans Comput 100:1100–1103, 1971
Chierichetti F, Pizzolato G: 18F-FDG-PET/CT. Q J Nucl Med Mol Imag 56:138–150, 2012
Vach W, Høilund-Carlsen PF, Gerke O, Weber WA: Generating evidence for clinical benefit of PET/CT in diagnosing cancer patients. J Nucl Med 52:77–85, 2011
Castellano G, Bonilha L, Li L, Cendes F: Texture analysis of medical images. Clin Radiol 59:1061–1069, 2004
Holli K, Lääperi A-L, Harrison L, Luukkaala T, Toivonen T, Ryymin P, Dastidar P, Soimakallio S, Eskola H: Characterization of breast cancer types by texture analysis of magnetic resonance images. Acad Radiol 17:135–141, 2010
Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V: Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 3:573–589, 2012
Ba-Ssalamah A, Muin D, Schernthaner R, Kulinna-Cosentini C, Bastati N, Stift J, Gore R, Mayerhoefer ME: Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol 82:537–543, 2013
Cheng N-M, Fang Y-HD, Yen T-C: The promise and limits of PET texture analysis. Ann Nucl Med 27:867–869, 2013
van Gómez López O, Vicente AMG, Martınez AFH, Soriano AM, Castrejón GAJL, Udias JM, León Atance P: Heterogeneity in [18 F] fluorodeoxyglucose positron emission tomography/computed tomography of non-small cell lung carcinoma and its relationship to metabolic parameters and pathologic staging. Mol Imaging 13:1–12, 2014
Sollini M, Cozzi L, Antunovic L, Chiti A, Kirienko M: PET Radiomics in NSCLC: state of the art and a proposal for harmonization of methodology. Sci Rep 7:358, 2017
Wu J, Aguilera T, Shultz D, Gudur M, Rubin DL, Loo BW, Diehn M, Li R: (2016) Early-Stage Non–Small Cell Lung Cancer: Quantitative Imaging Characteristics of 18F Fluorodeoxyglucose PET/CT Allow Prediction of Distant Metastasis. Radiology 281(1)
Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallières E, Wood DE: Lung cancer proliferation correlates with [F-18] fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 6:3837–3844, 2000
Cawley GC, Talbot NLC: Over-fitting in model selection and subsequent selection bias in performance evaluation. J Machine Learning Research 11:2079–2107, 2010
Hatt H, Tixier F, Pierce L, Kinahan PE, Rest CC, Visvikis D: (2016) Characterization of PET/CT images using texture analysis: the past, the present… any future? 44(1):151–165
Acknowledgements
This study was funded by TUBITAK (The Scientific and Technological Research Council of Turkey) under Project No.: 113E188.
Author information
Authors and Affiliations
Contributions
Contributing to conception and design: SK, BY, AT, OK
Acquiring data, or analyzing and interpreting data: SK, BY, OK, OA, EK, SI
Drafting the manuscript: SK, BY, OK, SI
Critically contributing to or revising the manuscript, or enhancing its intellectual content: SK, BY, EK, SI
Approving the final content of the manuscript: SK, BY, OK
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Karacavus, S., Yılmaz, B., Tasdemir, A. et al. Can Laws Be a Potential PET Image Texture Analysis Approach for Evaluation of Tumor Heterogeneity and Histopathological Characteristics in NSCLC?. J Digit Imaging 31, 210–223 (2018). https://doi.org/10.1007/s10278-017-9992-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10278-017-9992-3