Abstract
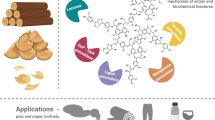
The breakdown of plant lignin modifies the structure of lignocelluloses, thus making carbohydrates accessible for efficient bioconversion. White-rot fungi produce ligninolytic enzymes such as lignin peroxidase, manganese peroxidase, laccases and various peroxidases, which mineralize lignin efficiently. We review here applications of ligninolytic enzymes for the delignification of lignocellulosic materials, the removal of recalcitrant organic pollutants, wastewater treatment, decolorization of dyes, soil treatment, conversion of high molecular weight coal fractions to low molecular weight coal fractions, which could be used as a feed stock for the production of commodity chemicals, biopulping and biobleaching in paper industries and enzymatic polymerization in polymer industries.
Similar content being viewed by others
References
Adam W, Lazarus M, Saha Moller CR, Weichold O, Hoch U, Haring D, Schreier P (1999) Biotransformations with peroxidases. Adv Biochem Eng Biotechnol 63:74–108
Akthar MN, Mohan PM (1995) Bioremediation of toxic metal ions from polluted lake waters and industrial effluents by fungal biosorbent. Curr Sci 69:1018–1030
Amitai G, Adami R, Moriah GS, Rabinovtz I, Vincze A, Leader H, Chefetz B, Leibovitz-Persky L, Friesem D, Hadar Y (1998) Oxidative biodegradation of phosphorothiolates by fungal laccase. FEBS Lett 438:195
Archibold F (1990) Decolourization of kraft bleachery effluent chromophores by coriolus (Trametes) versicolor. Enzyme Microb Technol 12:846–853
Bajpai P (1999) Application of enzymes in the pulp and paper industry. Biotechnol Prog 15:147
Bajpai P (2004) Biological bleaching of chemical pulps. Critical Rev in Biotechnol 24:1–58
Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30:215–242
Bogan BW, Lamar RT (1996) Polycyclic aromatic hydrocarbons degrading capabilities of Phenerochaete laevis HHB-1625 and its extracellular ligninolytic enzymes. Appl Environ Microbiol 62:1597–1603
Bogan BW, Lamar RT, Hammel KE (1996) Fluorene oxidation in vivo by Phanerochaete chrysosporium and in vitro during manganese peroxidase-dependent lipid peroxidation. Appl Environ Microbiol 62:1788–1792
Bollag JM (1992) Decontaminating soil with enzymes. Environ Sci Technol 26:1876
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates: an expanded role of laccase in lignin biodegradation. FEBS Lett 267:99–102
Breen A, Singleton FL (1999) Fungi in lignocellulose breakdown and biopulping. Current opinion in Biotechnol 10:252–258
Bumpus JA, Aust SD (1987) Biodegradation of DDT (1, 1, 1-trichloro-2,2-bis (4-chlorophenyl) ethane) by the white-rot fungus Phenerochaete chrysosporium. Appl Environ Microbiol 53:2001–2008
Bumpus JA, Tien M, Wright D, Aust SD (1985) Oxidation of persistent environmental pollutants by a white rot fungus. Science 228:1434–1436
Call HP, Mucke I (1997) History, overview and applications of mediated lignolytic systems, especially laccase-mediator systems (Lignozym(R)-Process). J Biotechnol 53:163–202
Camarero S, Bockle B, Martinez MJ, Martinez AT (1996) Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl Environ Microbiol 62:1070–1072
Cenek N, Katerina S, Pavla E, Tomas C, Aparna K, Elke L, Vaclav S (2004) Lignolytic fungi in bioremediation: extracellular enzyme production and degradation rate. Soil Biol Biochem 36:1545–1551
Chivukula M, Spadaro JT, Renganathan V (1995) Lignin peroxidase catalysed oxidation of sulfonated azo dyes generates novel sulfophenyl hydroperoxidases. Biochemistry 34:7765–7772
Christian V, Shrivastava R, Shukla D, Modi H, Rajiv B, Vyas M (2005) Mediator role of veratryl alcohol in the lignin peroxidase catalysed oxidative decolorization of Remazol Brilliant Blue R. Enzyme Microb Technol 36:426–431
Christov LP, Van Driessel B, Du Plessis CA (1999) Fungal biomass from Rhizomucor pulsillus as adsorbent of chromophores from a bleach plant effluent. Process Biochem 35:91–95
Cohen MS, Gabriele PB (1982) Degradation of coal by the fungi Polyporus versicplor and Poria monticola. Appl Environ Microbiol 44:23–27
Conto SR, Harrera JLT (2006) Industrial and biotechnological applications of laccase: a review. Biotecnol Adv 24:500–513
Crestini C, Argyropoulos DS (1998) The early oxidative biodegradation steps of residual kraft lignin models with laccase. Biorg Med Chem 6:2161
Dafale N, Nageswara RN, Sudhir U (2008) Decolorization of azodyes and simulated dye bath wastewater using acclimatized micro-bial consortium biostimulation and halo tolerance. Bioresour Technol 99:2552–2558
Dahiya JS, Singh D, Nigam P (1998) Characterization of laccase produced by Coniothyrium minitans. J Basic Microbiol 38:349
Davis JM, Lohmann RC, Phillips FM, Wilson JL, Love DW (1993) Architecture of the sierra ladrones formation, central new Mexico: depositional controls on the permeability correlation structure. Geol Soc Amer Bull 105(8):998–1007
DE Catcheside A, Ralph JP (1999) Biological processing of coal. Appl Microbiol Biotechnol 53:16–24
Dec J, Bollag JM (1994) Use of plant material for the decontamination of water polluted with phenols. Biotechnol Bioeng 44:1132
Dec J, Bollag JM (1995) Effect of various factors on dehalogenation of chlorinated phenols and anilines during oxidative coupling. Environ Sci Technol 29:657
Dunford HB (1991) Horseradish peroxidase: structure and kinetic properties. In: Everse J, Everse KE, Grisham MB (eds) Peroxidases in chemistry and biology. CRC Press, Boca Raton, pp 1–24
Duran N, Esposito E (2000) Potential applications of oxidative enzymes and phenoloxidase like compounds in waste water and soil treatment. Appl Catal B 28:83–89
Dwivedi UN, Singh P, Pandey VP, Kumar A (2011) Structure-function relationship among bacterial, fungal and plant laccases. J Mol catal B: Enzym 68:117–128
Dwran N, Minussi RC, Pastore GM, Alves OL, Gimenes IF, Peralta-zamora P, Moraes SG (2000) Laccase production and its environmental applications in the presence of mediators, In: CH Soares (eds), Proceedings of the second national meeting of environmental applied microbiology. Florianopolis, S.C. Brazil 2:12
Edwards SL, Raag R, Wariishi H, Michael HG, Thomas LP (1993) Crystal structure of lignin peroxidase. Proc Natl Acad Sci USA 90:750–754
Eibes G, Cajthaml T, Moreira MT, Feijoo G, Lema JM (2006) Enzymatic degradation of anthracene, dibenzothiophene and pyrene by manganese peroxidase in media containing acetone. Chemosphere 64:408–414
Eriksson KE, Kirk TK (1994) Biopulping: an overview of developments in an environmentally safe paper making technology. FEMS Microbiol Rev 13:351–364
Esposito E, Resende MOO, Freer J, Baeza J, Carvalho ME (1997) Ligninnolytic enzymes application on humic acid organochloride compounds association, in: E.H.M. Melo, M.C.B. Pimentel (Eds.), Proceedings of the Fourth Brazilian Symposium on Chemical Lignins other Wood Comp., Brazil, Vol. 5, 1995 p. 251. Chem Abstr. 126:108249
Esposito E, Manfio G, Villas-Boas S, Antunes R, Paulillo S, Souza JA (1997) Microbiological strategies on remediation of contaminated soil with organochlorides, In: Esposito E (ed) Proceedings of the first national meeting of environmental applied microbiology campinas, S.P., Brazil, (vol 1), p. 80. Chemical Abstract 127: 358427
Esposito E, Manfio G, Villas-Boas S, Manfio G (1998) Fungal potential for soil bioremediation, In: Gaylarde CC, Barbosa TCP, Gabilan NH (eds), Proceedings of the third latin american biodegradation and biodeterioration symposium-LABS-3, Florianopolis, Brazil, CD-Rom Paper 25
Ferrer I, Dezotti M, Duran N (1991) Decolorization of kraft effluent by free and immobilized lignin peroxidase and horseradish peroxidase. Biotechnol Lett 13:577–582
Field JA, De Jong E, Costa GF, De Bont JAM (1993) Screening of lignolytic fungi applicable to biodegradation of xenobiotics. Trends Biotechnol 11:44–49
Frichter M, Vares T, Kalsi M, Galkin S, Scheibner K, Fritsche W, Hatakka A (1999) Production of MnP and organic acids and mineralization of 14C-labelled lignin (14C-DHP) during solid-state fermentation of wheat-straw with the white rot fungus Nematoloma frowardii. Appl Environ Microbiol 65:1864–1870
Fritz-Langhals E, Kunath B (1998) Synthesis of aromatic aldehydes by laccase-mediater assisted oxidation. Tetrahedron Lett 39:5955–5956
Fujita M, Era A, Lke M, Soda S, Miyata N, Hirao T (2000) Decolorization of heat treatment liquor of waste sludge by a bioreactor using polyurethane foam immobilized white rot fungi equipped with an ultramembrane filtration unit. J Biosc Bioeng 90:387–394
Gavril M, Hodson PV (2007) Chemical evidence for the mechanism of the biodecoloration of Amaranth by Trametes versicolor. World J Microbiol Biotechnol 23:103–124
Gianfreda L, Sannina F, Filazzola MT, Leonowicz A (1998) Catalytic behaviour and detoxifying ability of a laccase from the fungal strain Cerrena unicolor. J Mol Catal B: Enzymatic 4:3
Glenn JK, Akileswaran L, Gold MH (1986) Mn(II) oxidation is the principal function of the extracellular Mn-Peroxidase from Phanerochaete chrysosporium. Arch Biochem Bioph 251:688–696
Grey R, Hafer C, Schlosser D (1998) Degradation of 2-chlorophenol and formation of 2-chloro-14-benzoquinone by mycelia and cell free crude culture liquids of Trametes versicolor in relation to extracellular laccase activity. J Basic Microbiol 38:371
Gunther T, Sack U, Hofrichter M, Latz M (1998) Oxidation of PAH and PAH-derivatives by fungal and plant oxidoreductases. J Basic Microbiol 38:113–122
Hammel KE, Cullen D (2008) Role of fungal peroxidases in biological ligninolysis. Curr opin in plant Biol 11:1–7
Hammel KS, Kalyanaraman B, Kirk TK (1986) Oxidation of polycyclic aromatic hydrocarbons and dibenzo[p]-dioxins by Phenerochaete chrysosporium ligninase. J Biol Chem 26:948–995
Harley BS, Brodo PMA, Senior PJ (1988) Proceeding of royal society discussion meeting on utilisation of lignocellulosic wastes. Cambridge Univ Press, Cambridge
Harvey PJ, Schoemaker HE, Palmer JM (1986) Veratryl alcohol as a mediator and the role of radical cations in lignin biodegradation by Phenerochaete chrysosporium. FEBS Lett 195:242–246
Hatakka A (1994) Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol Rev 13:125–135
Have RT, Rietjens IMCM, Hartmans S, Swarts HJ, Field JA (1998) Calculated ionization potentials determine the oxidation of vanillin precursors by lignin peroxidase. Federation of Eur Microbiol Soc Rev 13:125–135
Heinfling A, Ruiz-Duenas FJ, Martinez MJ, Bergbauer M, Szewzyk U, Martinez AT (1998) A study on reducing substrates of manganese oxidizing peroxidases from Pleurotus eryngii and Bjerkandera adusta. FEBS Lett 428:141–146
Hiroshi U, Shiro K (1999) Enzymatic polymerization yields useful polyphenols. Chemtech 29:22–28
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microb Technol 30:454–466
Ishihara A, Maeshima Y, Veta H, Oishi K (1997) Decolorization of dyes by laccase, Shizuoka-ken Hammatsu Kogyo Gijuku, Senta kenkyu Hokoku 721. Chem Abstr 128:4654
Isroi Rai M, Siti S, Claes N, Muhammad NC, Knut L, Mohammad JT (2011) Biological pretreatment of lignocelluloses with white rot fungi and its applications: a review. BioResources 6:5224–5259
Joshi DK, Gold MH (1994) Oxidation of dibenzo-p-dioxin by lignin peroxidase from the basidiomycete Phenerochaete chrysosporium. Biochemistry 33:1096–10976
Joshi SM, Inamalor SA, Telke AA (2010) Exploring the potential of natural bacterial consortium to degrade mixture of dyes and textile effluent. Int J Biodeter Biodegrad 64:622–628
Kadhim H, Graham C, Barratt P, Evans CS, Rastall RA (1999) Removal of phenolic compounds in water using Cariolus versicolor grown on wheat bran. Enzyme Microbiol Technol 24:303
Kang S, Shin KS, Han YH, Youn HD, Hah YC (1993) Purification and characterisation of an extracellular peroxidase from white-rot fungus Pleurotus ostreatus. Biochem Biophys Acta 1163:158–164
Kawai S, Nakagawa M, Ohashi H (1999) Aromatic ring cleavage a non-phenolic bête-O-4 lignin model dimmer by laccase of Trametes versicolor in the presence of 1-hydroxybenzotriazole. FEBS Lett 446:355
Kedderis GL, Hollenberg PF (1983) Steady–state kinetics of chloroperoxidase-catalyzed N-demethylation reactions. J Biol Chem 258:12413
Kersten PJ, Kalyanaraman B, Hammel KE, Reinhammar B, Kirk TK (1990) Comparison of lignin peroxidase, horseradish peroxidase and laccase in the oxidation of methoxybenzenes. Biochem J 268:475–480
Kirk TK, Cullen D (1998) Enzymology and molecular genetics of wood degradation by white-rot fungi. In: Young RA, Akthar M (eds) Environmentally friendly technologies for the pulp and paper industry. Wiley, New York, pp 273–307
Krikstopaitis K, Kulys J, Pederson AH, Schneider P (1998) N-substituted p-phenylenediamines as peroxidase and laccase substrates. Acta Chem Scan 52:469
Kurakale M, Ide N, Komaki T (2007) Biological pretreatment with two bacterial strains for enzymatic hydrolysis of office paper. Curr Microbiol 54:424–428
Kurek B, Petit-conil M, Sigoillot JC, Herpoel I, Ruel K, Moukha S, Joseleav JP, Pennincks M, Asther M,Gazza G and Dechoudens C (2001) Treatment of high yield pulp with fungal peroxidases from laboratory to pilot scale study.In: Argyropoulos D (ed) ACS Symposium Series 785, Oxidative delignification Chemistry, fundamental and catalysis. American Chemical Society, Washington DC. Chapter 30. 785:474–486
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Kwant SS, Chang JK (1998) Decolorisation of artificial dyes by peroxidase from the white-rot fungus Pleurotus ostreatus. Biotechnol Lett 20:569–572
Levin L, Papinutti L, Forchiassin F (2004) Evaluation of four Argentinean white rot fungi; their ability to produce lignin modifying enzymes and decolorize industrial dyes. Bioresour Technol 2:169–176
Lisov AV, Leontievsky AA, Golovleva LA (2003) Hybrid Mn-peroxidase from the ligninolytic fungus Panus tigrinus 8/18. Isolation, substrate specificity and catalytic cycle. Biochemistry (Moscow) 68:1027–1035
Maijala P,Mettalo A, Kleen M, Westin C, Poppius-Levin K,Herranen K, Lehto JH, Reponen P, Maentausta O and Hatakka A (2007) Treatment of softwood chips with enzymes may reduce refining energy consumption and increase surface charge of fibers. In: 10th international congress on Biotechnology in the pulp and paper Industry, Madison Wisconsin, Book of Abstracts. pp 65
Majcherczyk A, Johannes C, Huttermann A (1999) Oxidation of aromatic alcohols by laccase from Trametes versicolor mediated by 22′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) cation radical and dication. Appl Microbiol Biotechnol 51:267
Manzanares P, Fajardo S, Martin C (1995) Production of ligninolytic activities wheen treating paper pulp effluents by Trametes versicolor. J Biotechnol 4B:125–132
Marcia JMM, Ademir CES, Helena CTR (2010) Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. E J Biotechnol. doi:10.2225/vol13-issue6-fulltext-2
Martinez AT (2002) Molecular biology and structure and function of lignin-degrading heme peroxidases. Enzym Microb Technol 30:425–444
Martinez MJ, Martinez AT (1996) Characterization of MnP isoenzymes of Pleurotus eryngii exhibiting Mn-independent activities on 2,6-dimethoxyphenol and veratryl alcohol. In: Messner K, Srebotnik E (eds) Biotechnology in the pulp and paper industries: recent advances in applied and fundamental research. Facultas-Universitatsverlag, Vienna, pp 417–420
Martinez MJ, Ruiz-Duenas FJ, Guillen F, Martinez AT (1996) Purification and catalytic properties of two manganese-peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem 237:424–432
Martnez D, Larrondo LF, Putnam N, Gelpke MD, Huang K, Chapman J (2004) Genome sequence of the lignocellulose degrading fungus Phenerocheate chrysosporium strain RP78. Nature Biotechnol 22:695–700
Marwaha SS, Grover R, Prakash C, Kennedy JF (1998) Continuous biobleaching of black liquor from the pulp and paper industry using an immobilised cell system. J Chem Technol Biotechnol 73:292–296
May SW (1999) Applications of oxidoreductase. Curr Opin Biotechnol 10:370–375
Meera K, Yadav RSS, Yadav KDS (2002) Secretion of lignin peroxidase by Penicillium citrinum, Fusarium oxysporum and Aspergillus terreus. Indian J Expt Biol 40:802–806
Messerschmidt A (1997) Multi-copper oxidases. World Scientific, Singapore
Mester T, Field JA (1998) Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. J Biol Chem 273:15412–15417
Mikolasch A, Hammer E, Jones U, Popowski K, Stielow A, Schauer F (2002) Synthesis of 3-(3,4-dihydroxy phenyl)-propionic acid derivatives by N-coupling of amines using laccase. Tetrahedron 58:7589–7593
Milstein O, Huttermann A, Frund R, Ludemann HD (1994) Enzymatic copolymerization of lignin with low molecular mass compounds. Appl Miicrobiol Biotechnol 40:760–767
Moreira MT, Feijoo G, Canoval J, Lema JM (2003) Semipilot-scale bleaching of kraft pulp with MnP. Wood Sci and Technol 37:117–123
Nagarathnamma R, Bajpai P, Bajpai PK (1999) Studies on decolorization and detoxification of chlorinated lignin compounds in kraft bleaching effluents by Ceriporiopsis subvermispora. Process Biochem 34:939–948
Nilsson I, Moller A, Mattiasson B, Rubindamayugi MST, Welander U (2006) Decolorization of synthetic and real textile wastewater by the use of white-rot fungi. Enzyme Microb Technol 38:94–100
Ohmomo S, Kainuma M, Sirianuntapiboon S, Aoshima I, Atthasampunna P (1988) Adsorption of melanoidin to the mycelia of Aspergillus oryzae Y-32. Agric Biol Chem 52:381–386
Ozsoy HD, Unyayar A, Mazmanci MA (2005) Decolorization of reactive textile dyes Drimarene Blue X3LR and Remazol Brilliant Blue R by Funalia trogii ATCC 200800. Biodegradation 16:195–204
Palma C, Moreira MT, Mielgo I, Feijoo G, Lema IM (1999) Use of a fungal bioreactor as a post treatment step for continuous decolorisation of dyes. Water Sci Technol 40:131–136
Patel VK, Yadav RSS, Yadav KDS (2007) Enzymatic characteristics of lignin peroxidases of indigenous lignolytic fungal strains—part I Indian. J Biotechnol 6:553–556
Pelaez F, Martinez MJ, Martinez AT (1995) Screening of 68 species of basidiomycetes for enzymes involved in lignin degradation. Mycol Res 99:37–42
Perumal K, Kalaichelvan PT (1996) Production of extracellular lignin peroxidase and laccase by Ganoderma lucidum PTK3 on sugarcane bagasse lignin. Indian J Expt Biol 34:1121–1125
Pointing SB (2001) Feasibility of bioremediation by white-rot fungi. Appl Microbiol Biotechnol 57:20–33
Pothast A, Rosanau T, Koch H, Fisher K (1999) The reaction of phenolic model compounds in the laccase mediator system (LMS) investigations by matrix assisted laser desorption ionization time of flight mass spectrometry [MALDI-TOF-MS]. Holzfrschung 53:175
Quintanar L, Yoon J, Aznar CP, Palmer AE, Andersson KK, Britt RD, Solomon EI (2005) Spectroscopic and electronic structure studies of the trinuclear as cluster active site of the multicopper oxidase laccase: nature of its co-ordination unsaturation. J Am Chem Soc 127:13832–13845
Ralph JP, Catcheside DEA (1994) Decolourisation and depolymerisation of solubilised low-rank coal by the white-rot basidiomycete Phanerochaete chrysosporium. Appl Microbiol Biotechnol 42:536–542
Ralph JP, Graham LA, Catcheside DEA (1996) Extracellular oxidase and the transformation of solubilised fractions of low rank coal by wood rot fungi. Appl Microbiol Biotechnol 46:226
Rama R, Mougin C, Boyer FD, Kollmann A, Molosse C, Sigoillot JC (1998) Biotransformation of benzo[a]pyrene in bench scale reactor using laccase of Pycnoporus cinnabarinus. Biotechnol Lett 20:1101
Raper JC, Sarkar JM, Bollag JM (1995) Enhanced enzymatic removal of chlorophenols in the presence of co-substrates. Water Res 29:2720
Regalado C, Garcia-Almendarez BE, Duarte-Vazquez MA (2004) Biotechnological applications of peroxidases. Phytochem Rev 3:243–256
Rittstieg K, Suurnakki A, Suortti T, Kruus K, Guabitz AM, Buchart J (2003) Polymerisation of guaiacol and a phenolic β-o-4 substructure by Trametes hirsuta laccase in the presence of ABTS. Biotechnol Progess 19:1505–1509
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends in Biotechnol 24:219–226
Rodriguez S, Toca JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Ruiz-Duenas FJ, Martinez MJ, Martinez AT (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol 31:223–236
Saccomandi F, Conte P, Piccolo A (1998) Use of oxidase enzyme to increase polymerization of soil organic matter. Fresenius Environ Bull 7:537–543
Sack U, Hofrichter M, Fritsche W (1997) Degradation of polycyclic aromatic hydrocarbons by manganese peroxidase of Nematoloma frowardii. FEMS Microbiol Lett 152:227–234
Salvachua D, Prieto A, Lopez-Abelairas M, Lu-Chau T, Martinez AT, Martinez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Bioresour Technol 102:7500–7506
Sanchez C (2009) Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotech Adv. 27:185–194
Sanghi R, Dixit A, Guha S (2006) Sequential batch culture studies for the decolorization of reactive dye by Coriolus versicolor. Bioresour Technol 97:396–400
Sarkar S, Martinez AT, Martinez MJ (1997) Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochem Biophys Acta 1339:23–30
Sasek V (2003) Why mycoremediations have not yet come into practice. In: Sasek V, Glaser JA, Baveye P (eds) The utilization of bioremediation to reduce soil contamination: problems and solutions. Kluwer Academic Publishers, Amsterdam, pp 247–260
Satwinder SM, Rajesh G, Chand P, John FK (1998) Continuous biobleaching of black liquor from the pulp and paper industry using an immobilised cell system. J Chem Technol Biotechnol 73:292–296
Satyahari D, Maiti TK, Bhattacharyya BC (1994) Production of some extracellular enzymes by a lignin peroxidase-producing brown rot fungus, Polyporus ostreiformis, and its comparative abilities for lignin degradation and dye decolorization. Appl Environ Microbiol 60:4216–4218
Schweigert N, Zehnder AJB, Eggen RIL (2001) Chemical properties of catechols and their molecular modes of toxic action in cells from micro-organisms to mammals. Environ Microb 3:81–91
Shanmugan V, Yadav KDS (1996) Production of extracellular lignin peroxidase by Pleurotus sajor-caju. Indian J Expt Biol 34:1164–1165
Shanmugan V, Yadav KDS (1997) Production of lignin peroxidase by Rhizopus nigricans. Indian J Microbiol 37:105–106
Sharma JK, Yadav M, Singh NP, Yadav KDS (2011) Purification and characterisation of lignin peroxidase from Pycnoporus sanguineus MTCC-137. Appl Biochem Microbiol 47:532–537
Shuttleworth KL, Bollag JM (1986) Soluble and immobilized laccase as catalysts for the transformation of substituted phenols. Enzyme and Microbial Technol 8:171–177
Sigoillot C, Camarero S, Vidal T, Record E, Asther M, Colom JF, Martinez AT (2005) Comparison of different fungal enzymes for bleaching high-quality paper pulps. J of Biotechnol 115:333–343
Silva EM, Martins SF, Milagres AMF (2008) Extraction of manganese peroxidase produced by Lentinula edodes. Bioresour Technol 99:2471–2475
Strebotnik E, Hammel KE (2000) Degradation of nonphenolic lignin by the laccase/1-hidroxy benzotriazole system. J of Biotechnol 81:179–188
Sundaramoorthy M, Kishni K, Gold MH, Poulos TL (1994) The crystal structure of MnP from P.chrysosporium at 2.06-A resolution. J Biol Chem 269:32759–32767
Tanaka H, Koike K, Itakura S, Enoki A (2009) Degradation of wood and enzyme production by Ceriporiopsis subvermispora. Enzyme and Microb Technol 45:384–390
Tauber MM, Gubitz GM, Rehorek A (2008) Degradation of azo dyes by oxidative processes: laccase and ultrasound treatment. Biores Technol 99:4213–4220
Tein M, Kirk TK, Bull C, Fee JA (1986) Steady-state and transient-state kinetic studies on the oxidation of 3,4-dimethoxybenzyl alcohol catalyzed by the ligninase of Phanerocheate chrysosporium Burds. J Biol Chem 261:1687–1693
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161:238–249
Uan IC, Tien M (1993) Stimulation of Mn peroxidase activity: a possible role for oxalate in lignin biodegradation. Biochemistry 90:1242–1246
Valli K, Brock BJ, Joshi DK, Gold MH (1992) Degradation of 2,4-dinitrotoluene by the lignin- degrading fungus Phanerochaete chrysosporium. Appl Environ Microbiol 58:221–228
Van DB, Christov L (2002) Adsorption of colour from a bleach plant effluent using biomass and cell wall fractions from Rhizomucor pusillus. J Chem Technol Biotechnol 77:155–158
Vyas BRM, Vole J, Saaek V (1994) Ligninolytic enzymes of selected white-rot fungi cultivated on wheat-straw. Folia Microbiol 39:235–240
Wandrey C, Lies A, Kihumbu D (2000) Industrial biocatalysis: past, present and future. Org Proc Res Dev 4:285–290
Wang Y, Vazquez-Duhalt R, Pickard MA (2003) Manganese-lignin peroxidase hybrid from Bjerkandera adust oxidizes polycyclic aromatic hydrocarbons more activity in the absence of manganese. Can J Microbiol 49:675–682
Ward G, Hadar Y, Bilkis I, Dosoretz CG (2003) Mechanistic features of lignin peroxidase-catalysed oxidation of substituted phenols and 1,2-dimethoxyarenes. J Biol Chem 278:39726–39734
Wariishi H, Valli K, Gold MH (1991) In vitro depolymerization of lignin by MnP of Phanerochaete chrysosporium. Biochem Biophys Res Comm 176:269–275
Wariishi H, Valli K, Gold MH (1992) Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. Kinetic mechanism and role of chelators. J Biol Chem 267:23688–23695
Wasenberg O, Kryriakides I, Agathos SN (2003) White rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Advances 22:161–187
Widsten P, Kandelbauer A (2008) Laccase applications in the forest products industry; A review. Enzyme and Microbiol Technol 42:293–307
Wilson BW, Bean RM, Franz JA, Thomas BL, Cohen MS, Aronson H, Gray ET Jr (1987) Microbial conversion of low rank coal : characterisation of biodegraded product. Energy and Fuel 1:80–84
Xu F (1996) Oxidation of phenols, anilines and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry 35:7607–7614
Xu F (2005) Application of oxidoreductase: recent progress. Ind Biotechnol 1:38–50
Xu H, Lai YZ, Slomezynski D, Nakas JP, Tanenbaum SW (1997) Mediator-assisted selective oxidation of lignin model compounds by laccase from Botrytis anerea. Biotechnol Lett 19:957
Yadav M, Yadav KDS (2007) Lignocellulose biotechnology: future prospects. In: Kuhad RC, Singh A (eds). International publishing House Pvt. Ltd. New Delhi pp 63–88
Yadav M, Yadav P, Yadav KDS (2009a) Characterisation and coal depolymerizing activity of lignin peroxidase from Lenzitus seperia MTCC-1170. Biochemistry (Moscow) 74:1125–1131
Yadav M, Yadav P, Yadav KDS (2009b) Production, purification and characterisation of lignin peroxidase from Loweporus lividus MTCC-1178. Engg in life Sciences 9:124–129
Yadav M, Singh SK, Yadav KS, Yadav KDS (2010) Purification of lignin peroxidase from Hexagona tenuis MTCC-1119 and its kinetic properties in aqueous medium containing miscible organic solvents. Indian J Chem (Sec-B) 49B:489–494
Yadav M, Singh SK, Sharma JK, Yadav KDS (2011) Oxidation of polyaromatic hydrocarbons in system containing organic solvent by lignin peroxidase from Gleophyllum striatum MTCC-1117. Environ Technol 32:1287–1294
Yadav M, Singh SK, Yadava S (2012) Purification, characterisation and coal depolymerising activity of lignin peroxidase from Lenzitus betulina MTCC-1183. Appl Biochem and Microbiol 48:583–589
Yadav M, Singh SK, Yadav S,Yadav KDS (2015).Ligninolytic enzymes for water depollution, coal breakdown and paper industry In: E.Lichtfouse et al. (eds) CO2 sequestration, Biofuels and Depollution, in a series of book Environmental Chemistry for a Sustainable World, (vol 5). Springer International Publishing, Switzerland, pp 359–376 doi 10.1007/978-3-319-11906-9_10
Zhao X, Hardin IR (2007) HPLC and spectrophotometric analysis of biodegradation of azo dyes by Pleurotus ostreatus. Dyes Pigm 73:322–325
Zhao X, Yiping LY, Hardin I (2005) Determination of biodegradation products from sulfonated dyes by Pleurotus ostreatus using capillary electrophoresis coupled with mass spectrophoresis coupled with mass spectrometry. Biotechnol Lett 27:69–72
Zhao X, Hardin IR, Hwang HM (2006) Biodegradation of a model azo disperse dye by the white rot fungus Pleurotus ostreatus. Int Biodeterior Biodegrad 57:1–6
Zorn H, Langhoff S, Scheibner M, Nimtz M, Berger RG (2003) A peroxidase from Lepista irina cleaves ß, ß- carotene to flavor compounds. J Biol Chem 384:1049–1056
Acknowledgments
The financial support of DST, New Delhi, through its file no.SR/WOS-A/CS-138/2012 to Dr. Meera Yadav as a DST-Woman Scientist (WOS-A) is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yadav, M., Yadav, H.S. Applications of ligninolytic enzymes to pollutants, wastewater, dyes, soil, coal, paper and polymers. Environ Chem Lett 13, 309–318 (2015). https://doi.org/10.1007/s10311-015-0516-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-015-0516-4