Abstract
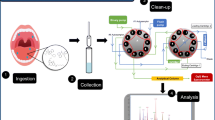
A liquid chromatography–tandem mass spectrometry method has been developed and validated for confirmation and quantification of three amphetamine-type stimulants (ATS) in oral fluid (OF), namely fenproporex (FEN), diethylpropione (DIE) and methylphenidate (MPH), which are misused by some Brazilian drivers and have potentially dangerous consequences in road traffic. In this method, the OF was extracted by a simple and fast liquid–liquid extraction using acetonitrile. Calibration curves were linear throughout the concentration range from 2.5 to 90 ng mL−1 in OF, using internal standard. The lower limit of quantification and the limit of detection were 2.5 and 0.5 ng mL−1, respectively, for all ATS. Intra- and inter-assay precision and accuracy values were within regulatory limits. The analyte extraction presented poor recoveries, but was considered appropriated for analyses of ATS in OF due to the low limits of detection. Matrix effects and carry over were not detected. All analytes were stable during the whole analytical procedure. The present analytical method was considered simple and sensitive for simultaneous determination of FEN, DIE, and MPH in OF and suitable for clinical and forensic toxicology.


Similar content being viewed by others
References
UNODC (United Nations Office obn Drugs Crime) (2011) Global ATS Assessment. Available online at: http://www.unodc.org/documents/ATS/ATS_Global_Assessment_2011.pdf
Pilgrim JL, Gerostamoulos D, Drummer OH, Bollmann M (2009) Involvement of amphetamines in sudden and unexpected death. J Forensic Sci 54:478–485
Leyton V, Sinagawa D, Oliveira K, Schmitz W, Andreuccetti G, De Martinis B, Yonamine M, Munoz D (2012) Amphetamine, cocaine and cannabinoids use among truck drivers on the roads in the State of Sao Paulo, Brazil. Forensic Sci Int 215:25–27
Myung SW, Min HK, Kim S, Kim M, Cho JB, Kim TJ (1998) Determination of amphetamine, methamphetamine and dimethamphetamine in Human Urine by Solid-Phase Microextraction (SPME)-Gas Chromatography/Mass Spectrometry. J Chromatogr B Biomed Sci Appl 716:359–365
Hjalmdahl M, Vadeby A, Forsman A, Fors C, Per Woxler GC, Kronstrand R (2012) Effects of d-amphetamine on simulated driving performance before and after sleep deprivation. Psychopharmac 222:401–411
Logan BK (2002) Methamphetamine—effects on human performance and behavior. Forens Sci Rev 14:133–151
Mariotti KC, Rossato LG, Froehlich PE, Limberger RP (2013) Amphetamine-type medicines: a review of pharmacokinetics, pharmacodynamics, and toxicological aspects. Curr Clin Pharmacol 8 (in press)
Nappo S, Tabach R, Noto A, Galduróz J, Carlini E (2002) Use of anorectic amphetamine-like drugs by Brazilian women. Eat Behav 3:153–165
Zancanaro I, Limberger RP, Bohel PO, Dos Santos MK, De Boni RB, Pechansky F, Caldas ED (2012) Prescription and illicit psychoactive drugs in oral fluid-LC-MS/MS method development and analysis of samples from Brazilian drivers. Forensic Sci Int 223:208–216
Drummer OH (2006) Drug Testing in Oral Fluid. Clin Biochem Rev 27:147–159
Concheiro M, Castro A, Quintela O, Cruz A, López-Rivadulla M (2007) Confirmation by LC-MS of drugs in oral fluid obtained from roadside testing. Forensic Sci Int 170:156–162
Schepers RJF, Oyler JM, Joseph RE Jr, Cone EJ, Moolchan ET, Huestis MA (2003) Methamphetamine and amphetamine pharmacokinetics in oral fluid and plasma after controlled oral methamphetamine administration to human volunteers. Clin Chem 49:121–132
Marchei E, Farrè M, Pellegrini M, García-Algar Ó, Vall O, Pacifici R, Pichini S (2010) Pharmacokinetics of methylphenidate in oral fluid and sweat of a pediatric subject. Forensic Sci Int 196:59–63
Pehrsson A, Gunnar T, Englom C, Seppä H, Jama A, Lillsunde P (2008) Roadside oral fluid test: comparison of the results of drugwipe 5 and drugwipe benzodiazepines on-site tests with laboratory confirmation results of oral fluid and whole blood. Forensic Sci Int 175:140–148
Crouch DJ, Walsh JM, Cangianelli L, Quintela O (2008) Laboratory evaluation and field application of roadside oral fluid collectors and drug testing devices. Ther Drug Monit 30:188–195
Comiran E, Souza DZ, Boehl PO, Mariotti KC, Pechansky F, Duarte PCAV, De Boni R, Froehlich PE, Limberger RP (2011) Fenproporex and amphetamine pharmacokinetics in oral fluid after controlled oral administration of fenproporex. Ther Drug Monit 34:545–553
Verstraete AG (2005) Oral fluid testing for driving under the influence of drugs: history, recent progress and remaining challenges. Forensic Sci Int 150:143–150
Meng P, Wang Y (2010) Small volume liquid extraction of amphetamines in saliva. Forensic Sci Int 197:80–84
Souza DZ, Boehl PO, Comiran E, Mariotti KC, Pechansky F, Duarte PCAV, De Boni R, Froehlich PE, Limberger RP (2012) Determination of amphetamine-type stimulants in oral fluid by solid-phase microextraction and gas chromatography mass spectrometry. Anal Chim Acta 696:67–76
Guidance for industry, bioanalytical of health and human services, food and drug administration (2001) Available: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. Accessed May 2001
Peters FT, Drummer OH, Musshoff F (2007) Validation of new methods. Forensic Sci Int 165:216–224
Romero-González R, Frenich AG, Vidal JLM, Prestes OD, Grio SL (2011) Simultaneous determination of pesticides, biopesticides and mycotoxins in organic products applying a quick, easy, cheap, effective, rugged and safe extraction procedure and ultra-high performance liquid chromatography—tandem mass spectrometry. J Chromatogr A 1218:1477–1485
Peters FT (2011) Recent advances of liquid chromatography–(tandem) mass spectrometry in clinical and forensic toxicology. Clin Biochem 44:54–65
McCalley DV (2010) The challenges of the analysis of basic compounds by high performance liquid chromatography: some possible approaches for improved separations. J Chromatogr A 1217:858–880
El Tayar N, van de Waterbeemd H, Testa B (1985) Measurements of protonated basic compounds by reversed-phase high-performance liquid chromatography: II. Procedure for the determination of a lipophilic index by reversed-phase high performance liquid chromatography. J Chromatogr 320:305–312
Namera A, Yashiki M, Kojima T (2002) Automated Headspace Solid-Phase Microextraction and In-Matrix Derivatization for the Determination of Amphetamine-Related Drugs in Human Urine by Gas Chromatography–Mass Spectrometry. J Chromatogr Sci 40:19–25
Maquille A, Guillarme D, Rudaz S, Veuthey JL (2009) High-throughput screening of drugs of abuse in urine by supported liquid–liquid extraction and uhplc coupled to Tandem MS. Chromatographia 70:1373–1380
Pal R, Megharaja M, Kirkbride KP, Heinrich T, Naidu R (2011) Biotic and abiotic degradation of illicit drugs, their precursor, and by-products in soil. Chemosphere 86:1002–1009
Raikos N, Christopoulou K, Theodoridis G, Tsoukali H, Psaroulis D (2003) Determination of amphetamines in human urine by headspace solid-phase microextraction and gas chromatography. J Chromatogr B Anal Technol Biomed Life Sci 789:59–63
Bosker WM, Huestis MA (2009) Oral fluid testing for drugs of abuse. Clin Chem 55(11):1910–1931
Taylor PJ (2005) Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry. Clin Biochem 38:328–334
Eeckhaut AV, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC-MS/MS assays: evaluation of matrix effects. J Chrom B 877:2198–2207
Cuadros-Rodríguez L, Bagur-González MG, Sánchez-Vinas M, González-Casado A, Gómez-Sáez AM (2007) Principles of analytical calibration/quantification for the separation sciences. J Chromatogr A 1158:33–46
Thompson M, Ellison SLR (2005) A review of interference effects and their correction in chemical analysis with special reference to uncertainty. Accred Qual Assur 10:82–97
Sulyok M, Krska R, Schuhmacher R (2007) A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal Bioanal Chem 389:1505–1523
Lopes RP, Reyes RC, Romero-Gonzalez R, Frenich AG, Vidal JLM (2012) Development and validation of a multiclass method for the determination of veterinary drug residues in chicken by ultra high performance liquid chromatography–tandem mass spectrometry. Talanta 89:201–208
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Bioanalytical method validation-a revisit with a decade of progress. Pharm Res 17:1551–1557
Peters FT, Maurer HH (2002) Bioanalytical method validation and its implications for forensic and clinical toxicology—a review. Accredit Qual Assur 7:441–449
Miller JN (1991) Basic statistical methods for Analytical Chemistry. Part 2. Calibration and regression methods: a review. Analyst 116:3–14
Acknowledgments
KCM thanks to CAPES-Brazil for her PhD grant. RPL thanks to CNPq for her research Grant. The authors thanks to LANAGRO/RS for kindly provide the opportunity of method development.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Cássia Mariotti, K., Rübensam, G., Barreto, F. et al. Simultaneous Determination of Fenproporex, Diethylpropione and Methylphenidate in Oral Fluid by LC-MS/MS. Chromatographia 77, 83–90 (2014). https://doi.org/10.1007/s10337-013-2569-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-013-2569-5