Abstract
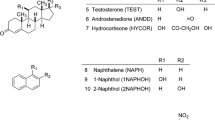
The ratio between the volume of the stationary phase and the void volume of column in HPLC is known as the phase ratio (denoted by Φ). In almost all the thermodynamic studies based on van’t Hoff plots for estimating the values of standard enthalpy and standard entropy, the phase ratio of HPLC column is assumed to be temperature invariant. The validity of this assumption is examined in the present work by studying the variation of Φ with temperature (T) in the range of 20–50 °C for three commercially available C18 columns and two mobile phase compositions methanol/water. A procedure based on proportionality of retention factors k on octanol/water partition coefficients log Kow for several aromatic hydrocarbon homologues was used for the measurement of Φ. Variation of Φ with temperature was previously reported for RP-HPLC separations in an acetonitrile/water mobile phase. However, while for acetonitrile/water mobile phase the effective value of Φ is decreasing as the temperature increases, in the case of methanol/water mobile phase the variation is more complicated. In some cases Φ is decreasing similar to the case of acetonitrile/water mobile phase, but in other cases it decreases up to a point and then shows a slight increase. The study proves that the findings regarding variability of Φ with temperature for the case of acetonitrile/water mobile phase is not an unique effect. In addition, present study evaluates how much the calculation of standard enthalpy and entropy from van’t Hoff plots differ when the variation of Φ with temperature is taken into consideration as compared to the classic approach when Φ is considered a constant.



Similar content being viewed by others
References
Wang M, Mallette J, Parcher JF (2009) Fundamental mathematical relationships for retention volumes in liquid chromatography. J Chromatogr A 1213(2):105–109
Yun KS, Zhu C, Parcher JF (1995) Theoretical relationships between the void volume, mobile phase volume, retention volume, adsorption, and Gibbs free energy in chromatographic processes. Anal Chem 67(3):613–619
Moldoveanu SC, David V (2013) Essentials in modern HPLC separations. Elsevier, Amsterdam, p 58
Jandera P (1993) Correlation of retention and selectivity of separation in reversed-phase high-performance liquid chromatography with interaction indices and with lipophilic and polar structural indices. J Chromatogr A 656(1–2):437–467
Kaune A, Knorrenschild M, Kettrup A (1995) Predicting 1-octanol-water partition coefficient by high-performance liquid chromatography gradient elution. Fresenius J Anal Chem 352(3–4):303–312
Valko K, Bevan C, Reynolds D (1997) Chromatographic hydrophobicity index by fast-gradient RP-HPLC: a high-throughput alternative to log P/log D. Anal Chem 69(11):2022–2029
Liang C, Qiao J-Q, Lian H-Z (2017) Determination of reversed-phase high performance liquid chromatography based octanol-water partition coefficients for neutral and ionizable compounds: methodology evaluation. J Chromatogr A 1528:25–34
Moldoveanu SC, Caiali E, David V (2017) Phase ratio and equilibrium constant in RP-HPLC obtained from octanol/water partition constant through solvophobic theory. Chromatographia 80(1–2):1491–1500
Rimmer CA, Simmons CR, Dorsey JG (2002) The measurement and meaning of void volumes in reversed-phase liquid chromatography. J Chromatogr A 965(1–2):219–232
Alhedai A, Martire DE, Scott RPW (1989) Column dead volume in liquid chromatography. Analyst 114(8):869–875
Wang M, Mallette J, Parcher JF (2011) Comparison of void volume mobile phase volume accessible volume determined from retention data for oligomers in reversed-phase liquid chromatographic systems. J Chromatogr A 1218(20):2995–3001
Sentell KB, Dorsey JG (1988) On the calculation of the stationary phase volume in reversed phase chromatography. J Liq Chromatogr 11(9–10):1875–1885
Bocian S, Vajda P, Felinger A, Buszewski B (2008) Solvent excess adsorption on the stationary phases for reversed-phase liquid chromatography with polar functional groups. J Chromatogr A 1204(1):35–41
Dorsey JG, Dill KA (1989) The molecular mechanism of retention in reversed-phase liquid chromatography. Chem Rev 89(2):331–346
Žuvela P, Skoczylas M, Liu JJ, Ba̧czek T, Kaliszan R, Wong MW, Buszewski B, (2019) Column characterization and selection systems in reversed-phase high-performance liquid chromatography. Chem Rev 119(6):3674–3729
Moldoveanu S, David V (2015) Estimation of phase ratio in reversed-phase high performance liquid chromatography. J Chromatogr A 1381:194–201
Caiali E, David V, Aboul-Enein HY, Moldoveanu SC (2016) Evaluation of the phase ratio for three C18 high performance liquid chromatographic columns. J Chromatogr A 1435:85–91
Caiali E, Moldoveanu SC, David V (2017) Comparison of the phase ratio for C18 HPLC columns using three different organic modifiers (methanol, ethanol, and acetonitrile) in mobile phase composition. Rev Roum Chim 62(8–9):629–636
Sangawitayakorn C, Wilairat P, Chantiwas R (2020) Experimental determination of phase ratio of C8 columns employing retention factors and octane-mobile phase partition coefficients of homologous series of linear alkylbenzenes. J Chromatogr A 1634:461668
Bocian S, Soukup J, Jandera P, Buszewski B (2014) Thermodynamics study of solvent adsorption on octadecyl-modified silica. Chromatographia 78(1):21–30
Buszewski B, Bocian S, Zera R (2010) Influence of temperature and pressure on the preferential adsorption of component of hydroorganic mobile phase in liquid chromatography. Adsorption 16(4):437–445
Buszewski B, Bocian S, Rychlicki G, Vajda P, Felinger A (2010) Study of solvent adsorption on chemically bonded stationary phases by microcalorimetry and liquid chromatography. J Colloid Interface Sci 349(2):620–625
Vailaya A (2005) Fundamentals of reversed phase chromatography: thermodynamic and exothermodynamic treatment. J Liq Chromatogr Rel Technol 28(7–8):965–1054
Kasturi P, Buszewski B, Jaroniec M, Gilpin RK (1994) Reordering/resonation studies of alkylamide phases. J Chromatogr A 659(2):261–265
Chester TL, Coym JW (2003) Effect of phase ratio on van’t Hoff analysis in reversed-phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy. J Chromatogr A 1003(1–2):101–111
Soare A-C, David V, Moldoveanu SC (2020) Does phase ratio in reversed phase high performance liquid chromatography vary with temperature? J Chromatogr A 1620:461023
Jaroniec M (1993) Partition and displacement models in reversed-phase liquid chromatography with mixed eluents. J Chromatogr A 656(1–2):37–50
Morel D, Serpinet J (1980) Gas chromatographic evidence for phase transitions in very compact octadecyl bonded silicas. J Chromatogr A 200(1):95–104
David V, Moldoveanu SC (2019) Variation with temperature of octanol/water partition coefficient for the homologous series from benzene to propylbenzene. Sep Sci Plus 2(12):457–464
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soare, AC., David, V. & Moldoveanu, S.C. Variation with Temperature of Phase Ratio in Reversed Phase HPLC for a Methanol/Water Mobile Phase. Chromatographia 84, 581–587 (2021). https://doi.org/10.1007/s10337-021-04038-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-021-04038-7