Abstract
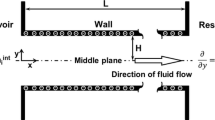
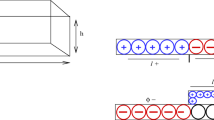
Considering the wide applications of the electrokinetic flow regulated by pH, we model the flow of an electrolyte solution containing multiple ionic species in a charge-regulated cylindrical nanochannel. This extends previous analyses, where only two kinds of ionic species are usually considered, and a charged surface assumed to maintain at either constant potential or constant charge density, to a case closer to reality. Adopting a fused silica channel containing an aqueous NaCl background salt solution with its pH adjusted by HCl and NaOH as an example, we show that if the density of the functional groups on the channel surface increases (decreases), it approaches a constant potential (charge density) surface; if that density is low, the channel behavior is similar to that of a constant charge density channel at high salt concentration and large channel radius. Several interesting results are observed, for example, the volumetric flow rate of a small channel has a local maximum as salt concentration varies, which is not seen in a constant potential or charge density channel.
Graphical Abstract












Similar content being viewed by others
References
Ai Y, Liu J, Zhang BK, Qian S (2010) Field effect regulation of DNA translocation through a nanopore. Anal Chem 82:8217–8225
Campbell LC, Wilkinson MJ, Manz A, Camilleri P, Humphreys CJ (2004) Electrophoretic manipulation of single DNA molecules in nanofabricated capillaries. Lab Chip 4:225–229
Cao QQ, Zuo CC, Li LJ, Ma YH, Li N (2010) Electroosmotic flow in a nanofluidic channel coated with neutral polymers. Microfluid Nanofluid 9:1051–1062
Chen Z, Ping Wang, Chang HC (2005) An electro-osmotic micro-pump based on monolithic silica for micro-flow analyses and electro-sprays. Anal Bioanal Chem 382:817–824
Chu HCW, Ng CO (2012) Electroosmotic flow through a circular tube with slip–stick striped wall. J Fluids Eng 134:111201
Daiguji H, Yang PD, Majumdar A (2004a) Ion transport in nanofluidic channels. Nano Lett 4:137–142
Daiguji H, Yang PD, Szeri AJ, Majumdar A (2004b) Electrochemomechanical energy conversion in nanofluidic channels. Nano Lett 4:2315–2321
Dolnik V (2004) Wall coating for capillary electrophoresis on microchips. Electrophoresis 25:3589–3601
Gasparac R, Kohli P, Mota MO, Trofin L, Martin CR (2004) Template synthesis of nano test tubes. Nano Lett 4:513–516
Hirst LS, Parker ER, Abu-Samah Z, Li Y, Pynn R, MacDonald NC, Safinya CR (2005) Microchannel systems in titanium and silicon for structural and mechanical studies of aligned protein self-assemblies. Langmuir 21:3910–3914
Hsu JP, Hung SH, Yu HY (2004a) Electrophoresis of a sphere at an arbitrary position in a spherical cavity filled with Carreau fluid. J Colloid Interface Sci 280:256–263
Hsu JP, Ku MH, Kao CY (2004b) Electrophoresis of a spherical particle along the axis of a cylindrical pore: effect of electroosmotic flow. J Colloid Interface Sci 276:248–254
Hu K, Bard AJ (1997) Characterization of adsorption of sodium dodecyl sulfate on charge-regulated substrates by atomic force microscopy force measurements. Langmuir 13:5418–5425
Karenga S, El Rassi Z (2010) A novel, neutral hydroxylated octadecyl acrylate monolith with fast electroosmotic flow velocity and its application to the separation of various solutes including peptides and proteins in the absence of electrostatic interactions. Electrophoresis 31:3192–3199
Karnik R, Castelino K, Fan R, Yang P, Majumdar A (2005) Effects of biological reactions and modifications on conductance of nanofluidic channels. Nano Lett 5:1638–1642
Kim SJ, Wang YC, Lee JH, Jang H, Han J (2007) Concentration polarization and nonlinear electrokinetic flow near a nanofluidic channel. Phys Rev Lett 99:044501
Kirby BJ, Hasselbrink EF (2004) Zeta potential of microfluidic substrates: 1. Theory, experimental techniques, and effects on separations. Electrophoresis 25:187–202
Krapf D, Wu MY, Smeets RMM, Zandbergen HW, Dekker C, Lemay SG (2006) Fabrication and characterization of nanopore-based electrodes with radii down to 2 nm. Nano Lett 6:105–109
Li J, Stein D, McMullan C, Branton D, Aziz MJ, Golovchenko JA (2001) Ion-beam sculpting at nanometer length scales. Nature 412:166–169
Liu CC, Maciel GE (1996) The fumed silica surface: a study by NMR. J Am Chem Soc 118:5103–5119
Mao P, Han J (2005) Fabrication and characterization of 20 nm planar nanofluidic channels by glass–glass and glass–silicon bonding. Lab Chip 5:837–844
Mei J, Xu JR, Xiao YX, Liao XY, Qiu GF, Feng YQ (2008) A novel covalent coupling method for coating of capillaries with liposomes in capillary electrophoresis. Electrophoresis 29:3825–3833
Nawrocki J (1997) The silanol group and its role in liquid chromatography. J Chromatogr A 779:29–71
Nischang I, Chen G, Tallarek U (2006) Electrohydrodynamics in hierarchically structured monolithic and particulate fixed beds. J Chromatogr A 1109:32–50
O’Brien RW, White LR (1978) Electrophoretic mobility of a spherical colloidal particle. J Chem Soc Faraday Trans 2 74:1607–1626
Ohshima H (1995) Electrophoresis of soft particles. Adv Colloid Interface Sci 62:189–235
Ohshima H (2006) Electrophoresis of soft particles: analytic approximations. Electrophoresis 27:526–533
Pennathur S, Santiago JG (2005) Electrokinetic transport in nanochannels. 1. Theory. Anal Chem 77:6772–6781
Pu QS, Yun JS, Temkin H, Liu SR (2004) Ion-enrichment and ion-depletion effect of nanochannel structures. Nano Lett 4:1099–1103
Qiao R, Aluru NR (2004) Charge inversion and flow reversal in a nanochannel electro-osmotic flow. Phys Rev Lett 92:198301
Qu WL, Li DQ (2000) A model for overlapped EDL fields. J Colloid Interface Sci 224:397–407
Schoch RB, Bertsch A, Renaud P (2006) pH-controlled diffusion of proteins with different pI values across a nanochannel on a chip. Nano Lett 6:543–547
Storm AJ, Chen JH, Ling XS, Zandbergen HW, Dekker C (2003) Fabrication of solid-state nanopores with single-nanometre precision. Nat Mater 2:537–540
Tas N, Berenschot J, Mela P, Jansen H, Elwenspoek M, van den Berg A (2002) 2D-confined nanochannels fabricated by conventional micromachining. Nano Lett 2:1031–1032
Thompson AP (2003) Nonequilibrium molecular dynamics simulation of electro-osmotic flow in a charged nanopore. J Chem Phys 119:7503–7511
van der Heyden FHJ, Stein D, Dekker C (2005) Streaming currents in a single nanofluidic channel. Phys Rev Lett 95:116104
Wang M, Liu J, Chen S (2007) Electric potential distribution in nanoscale electroosmosis: from molecules to continuum. Mol Simul 33:1273–1277
Wang XY, Wang SL, Gendhar B, Cheng C, Byun CK, Li GB, Zhao MP, Liu SR (2009) Electroosmotic pumps for microflow analysis. Trends Anal Chem 28:64–74
Wang M, Kang QJ, Ben-Naim E (2010) Modeling of electrokinetic transport in silica nanofluidic channels. Anal Chim Acta 664:158–164
White HS, Bund A (2008) Ion current rectification at nanopores in glass membranes. Langmuir 24:2212–2218
Yeh LH, Hsu JP, Qian S, Tseng SJ (2012a) Counterion condensation in pH-regulated polyelectrolytes. Electrochem Commun 19:97–100
Yeh LH, Xue S, Joo SW, Qian S, Hsu JP (2012b) Field effect regulation of surface charge property and EOF in nanofluidics. J Phys Chem C 116:4209–4216
Yuan Z, Garcia AL, Lopez GP, Petsev DN (2007) Electrokinetic transport and separations in fluidic nanochannels. Electrophoresis 28:595–610
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tseng, S., Tai, YH. & Hsu, JP. Electrokinetic flow in a pH-regulated, cylindrical nanochannel containing multiple ionic species. Microfluid Nanofluid 15, 847–857 (2013). https://doi.org/10.1007/s10404-013-1185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-013-1185-x