Abstract
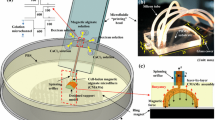
Combining microfluidic methods for generating microdroplets and spinning microfibers, a novel type of alginate hybrid microfiber encapsulating different microdroplets is fabricated for various applications such as cell culture, tissue engineering and drug release. Traditional fabrication methods mainly depend on the microfluidic structure, so an effective method that uses microfluidic solution flow rates to control the generation of hybrid microfibers has not yet been developed. In this paper, we fabricate a microfluidic flow-focusing device with a long gelation microchannel to encapsulate magnetic oil microdroplets (MOMs) into alginate microfibers. We establish a hybrid microfiber generation model for this fabrication method based on limited flow rate to control microfiber width, MOM diameter and the distance between consecutive MOMs. We also calculate the magnetic force acting on a single MOM by measuring the distance and the MOM is deflected by disk magnets with respect to time in the long gelation microchannel. The magnetic forces acting on the microfibers can be further calculated by counting the number of encapsulated MOMs. The developed method has great potential for quantitative fabrication of diverse hybrid microfibers encapsulating a variety of magnetic hydrophobic microdroplets with estimated magnetic forces. Such magnetic hybrid microfibers are attractive for use in higher order alginate microfiber assemblies and dual drug delivery systems.






Similar content being viewed by others
References
Agarwal P, Zhao ST, Bielecki P, Rao W, Choi JK, Zhao Y, Yu JH, Zhang WJ, He XM (2013) One-step microfluidic generation of pre-hatching embryo-like core-shell microcapsules for miniaturized 3D culture of pluripotent stem cells. Lab Chip. doi:10.1039/c3lc50678a
Atencia J, Beebe DJ (2005) Controlled microfluidic interfaces. Nature 437:648–655. doi:10.1038/nature04163
Christopher GF, Anna SL (2007) Microfluidic methods for generating continuous droplet streams. J Phys D Appl Phys 40:319–336. doi:10.1088/0022-3727/40/19/R01
Ghorbanian S, Qasaimeh MA, Akbari M, Tamayol A, Juncker D (2014) Microfluidic direct writer with integrated declogging mechanism for fabricating cell-laden hydrogel constructs. Biomed Microdevices. doi:10.1007/s10544-014-9842-8
Hanuš J, Ullrich M, Dohnal J, Singh M, Štěpánek F (2013) Remotely controlled diffusion from magnetic liposome microgels. Langmuir 29:4381−4387. doi.org/10.1021/la4000318
Hu CZ, Nakajima M, Yue T, Takeuchi M, Seki M, Huang Q, Fukuda T (2013) On-chip fabrication of magnetic alginate hydrogel microfibers by multilayered pneumatic microvalves. Microfluid Nanofluidics. doi:10.1007/s10404-013-1325-3
Jose MB, Cubaud T (2012) Droplet arrangement and coalescence in diverging/converging microchannels. Microfluid Nanofluidics 12:687–696. doi:10.1007/s10404-011-0909-z
Kang E, Jeong GS, Choi YY, Lee KH, Khademhosseini A, Lee SH (2011) Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat Mater 10:877–883. doi:10.1038/NMAT3108
Kang E, Choi YY, Chae SK, Moon JH, Chang JY, Lee SH (2012) Microfluidic spinning of flat alginate fibers with grooves for cell-aligning scaffolds. Adv Mater 24:4271–4277. doi:10.1002/adma.201201232
Lee CB, Chang CC, Huang SB, Yang RJ (2006) The hydrodynamic focusing effect inside rectangular microchannels. J Micromech Microeng 16:1024–1032
Li YH, Huang GY, Zhang XH, Li BQ, Chen YM, Liu TL, Lu TJ, Xu F (2013) Magnetic hydrogels and their potential biomedical applications. Adv Funct Mater 23:660–672. doi:10.1002/adfm.201201708
Lin YS, Huang KS, Yang CH, Wang CY, Yang YS, Hsu HC, Liao YJ, Tsai CW (2012) Microfluidic synthesis of microfibers for magnetic-responsive controlled drug release and cell culture. PLoS One. doi:10.1371/journal.pone.0033184
Liu HH, Zhang YH (2011) Droplet formation in microfluidic cross-junctions. Phys Fluids 23:987–999
Liu J, Shi J, Zhang F, Wang L, Yamamoto S, Takano M, Lianmei J, Haoli Z (2012) Segmented magnetic nanofibers for single cell manipulation. Appl Surf Sci 258:7530–7535. doi:10.1016/j.apsusc.2012.04.077
Miller E, Rotea M, Rothstein JP (2010) Microfluidic device incorporating closed loop feedback control for uniform and tunable production of micro-droplets. Lab Chip 10:1293–1301. doi:10.1039/b925497h
Shin SJ, Park JY, Lee JY, Park H, Park YD, Lee KB, Whang CM, Lee SH (2007) “On the fly” Continuous Generation of alginate fibers using a microfluidic device. Langmuir 23:9104–9108
Suh SK, Chapin SC, Hatton TA, Doyle PS (2012) Synthesis of magnetic hydrogel microparticles for bioassays and tweezer manipulation in microwells. Microfluid Nanofluidics 13:665–674. doi:10.1007/s10404-012-0977-8
Sun RP, Cubaud T (2011) Dissolution of carbon dioxide bubbles and microfluidic multiphase flows. Lab Chip 11:2924–2928. doi:10.1039/c1lc20348g
Thomas A, Gilson CD, Ahmed T (1995) Gelling of alginate fibres. J Chem Technol Biotechnol 64:73–79
Thorsen T, Roberts RW, Arnold FH, Quake SR (2001) Dynamic pattern formation in a vesicle-generating microfluidic device. Phys Rev Lett 86:4163–4166. doi:10.1103/PhysRevLett.86.4163
Vladisavljević GT, Kobayashi I, Nakajima M (2012) Production of uniform droplets using membrane, microchannel and microfluidic emulsification devices. Microfluid Nanofluidics 13:151–178. doi:10.1007/s10404-012-0948-0
Xu F, Wu MCA, Rengarajan V, Finley TD, Keles HO, Sung Y, Li BQ, Gurkan UA, Demirci U (2011) Three-dimensional magnetic assembly of microscale hydrogels. Adv Mater 23:4254–4260. doi:10.1002/adma.201101962
Yamada M, Sugaya S, Naganuma Y, Seki M (2012) Microfluidic synthesis of chemically and physically anisotropic hydrogel microfibers for guided cell growth and networking. Soft Matter 8:3122–3130
Yang K, Peng HB, Wen YH, Li N (2009a) Re-examination of characteristic FTIR spectrum of secondary layer in bilayer oleic acid-coated Fe3O4 nanoparticles. Appl Surf Sci 256:3093–3097. doi:10.1016/j.apsusc.2009.11.079
Yang CH, Huang KS, Lin YS, Lu K, Tzeng CC, Wang EC, Lin CH, Hsu WY, Chang JY (2009b) Microfluidic assisted synthesis of multi-functional polycaprolactone microcapusles: incorporation of CdTe quantum dots, Fe3O4 superparamagnetic nanoparticles and tamoxifen anticancer drugs. Lab Chip 9:961–965. doi:10.1039/b814952f
Yu Y, Wen H, Ma JY, Lykkemark S, Xu H, Qin JH (2014) Flexible fabrication of biomimetic bamboo-like hybrid microfibers. Adv Mater 26:2494–2499. doi:10.1002/adma.201304974
Zhang K, Liang QL, Ma S, Mu X, Hu P, Wang YM, Luo G (2009) On-chip manipulation of continuous picoliter-volume superparmagnetic dorplets using a magnetic force. Lab Chip 9:2992–2999
Acknowledgments
We thank Hasegawa lab at Nagoya University for the help during the design of microfluidic device. This work is supported by the National Natural Science Foundation of China under Grant Nos: 61375108, 61433010 and “111 Project” under Grant No: B08043.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, T., Hu, C., Nakajima, M. et al. On-chip fabrication and magnetic force estimation of peapod-like hybrid microfibers using a microfluidic device. Microfluid Nanofluid 18, 1177–1187 (2015). https://doi.org/10.1007/s10404-014-1511-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-014-1511-y