Abstract
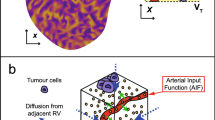
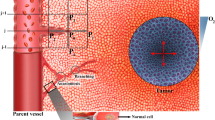
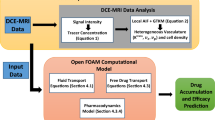
An MR image-based computational model of a murine KHT sarcoma is presented that allows the calculation of plasma fluid and solute transport within tissue. Such image-based models of solid tumors may be used to optimize patient-specific therapies. This model incorporates heterogeneous vasculature and tissue porosity to account for nonuniform perfusion of an MR-visible tracer, gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA). Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was conducted following intravenous infusion of Gd-DTPA to provide 1 h of tracer-concentration distribution data within tissue. Early time points (19 min) were used to construct 3D K trans and porosity maps using a two-compartment model; tracer transport was predicted at later time points using a 3D porous media model. Model development involved selecting an arterial input function (AIF) and conducting a sensitivity analysis of model parameters (tissue, vascular, and initial estimation of solute concentration in plasma) to investigate the effects on transport for a specific tumor. The developed model was then used to predict transport in two additional tumors. The sensitivity analysis suggests that plasma fluid transport is more sensitive to parameter changes than solute transport due to the dominance of transvascular exchange. Gd-DTPA distribution was similar to experimental patterns, but differences in Gd-DTPA magnitude at later time points may result from inaccurate selection of AIF. Thus, accurate AIF estimation is important for later time point prediction of low molecular weight tracer or drug transport in smaller tumors.









Similar content being viewed by others
References
Aref, M., M. Brechbiel, and E. C. Wiener. Identifying tumor vascular permeability heterogeneity with magnetic resonance imaging contrast agents. Invest. Radiol. 37:178–192, 2002.
Baudelet, C., R. Ansiaux, B. F. Jordan, X. Havaux, B. Macq, and B. Gallez. Physiological noise in murine solid tumours using T2*-weighted gradient-echo imaging: a marker of tumour acute hypoxia? Phys. Med. Biol. 49:3389–3411, 2004.
Baxter, L. T., and R. K. Jain. Transport of fluid and macromolecules in tumors. 1. Role of interstitial pressure and convection. Microvasc. Res. 37:77–104, 1989.
Baxter, L. T., and R. K. Jain. Transport of fluid and macromolecules in tumors. 2. Role of heterogeneous perfusion and lymphatics. Microvasc. Res. 40:246–263, 1990.
Boucher, Y., L. T. Baxter, and R. K. Jain. Interstitial pressure-gradients in tissue-isolated and subcutaneous tumors—implications for therapy. Cancer Res. 50:4478–4484, 1990.
Boucher, Y., and R. K. Jain. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 52:5110–5114, 1992.
Boucher, Y., J. M. Kirkwood, D. Opacic, M. Desantis, and R. K. Jain. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res. 51:6691–6694, 1991.
Chang, S. F., C. A. Chang, D. Y. Lee, P. L. Lee, Y. M. Yeh, C. R. Yeh, C. K. Cheng, S. Chien, and J. J. Chiu. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc. Natl. Acad. Sci. USA 105:3927–3932, 2008.
Chen, X. M., G. W. Astary, H. Sepulveda, T. H. Mareci, and M. Sarntinoranont. Quantitative assessment of macromolecular concentration during direct infusion into an agarose hydrogel phantom using contrast-enhanced MRI. Magn. Reson. Imaging 26:1433–1441, 2008.
Deng, J., T. K. Rhee, K. T. Sato, R. Salem, K. Haines, T. Paunesku, M. F. Mulcahy, F. H. Miller, R. A. Omary, and A. C. Larson. In vivo diffusion-weighted imaging of liver tumor necrosis in the VX2 rabbit model at 1.5 Tesla. Invest. Radiol. 41:410–414, 2006.
Diehl, K. H., R. Hull, D. Morton, R. Pfister, Y. Rabemampianina, D. Smith, J. M. Vidal, and C. van de Vorstenbosch. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 21:15–23, 2001.
Dreher, M. R., W. G. Liu, C. R. Michelich, M. W. Dewhirst, F. Yuan, and A. Chilkoti. Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer I 98:335–344, 2006.
Eikenberry, S. A tumor cord model for doxorubicin delivery and dose optimization in solid tumors. Theor. Biol. Med. Model. 6:16, 2009.
Elkareh, A. W., and T. W. Secomb. Effect of increasing vascular hydraulic conductivity on delivery of macromolecular drugs to tumor cells. Int. J. Radiat. Oncol. Biol. Phys. 32:1419–1423, 1995.
Foy, B. D., and J. Blake. Diffusion of paramagnetically labeled proteins in cartilage: enhancement of the 1-D NMR imaging technique. J. Magn. Reson. 148:126–134, 2001.
Furman-Haran, E., D. Grobgeld, and H. Degani. Dynamic contrast-enhanced imaging and analysis at high spatial resolution of MCF7 human breast tumors. J. Magn. Reson. 128:161–171, 1997.
Fyles, A., M. Milosevic, M. Pintilie, and R. P. Hill. Long-term performance of hypoxia and IFP as prognostic factors in cervix cancer. Radiother. Oncol. 78:67, 2006.
Gordon, M. J., K. C. Chu, A. Margaritis, A. J. Martin, C. R. Ethier, and B. K. Rutt. Measurement of Gd-DTPA diffusion through PVA hydrogel using a novel magnetic resonance imaging method. Biotechnol. Bioeng. 65:459–467, 1999.
Gutmann, R., M. Leunig, J. Feyh, A. E. Goetz, K. Messmer, E. Kastenbauer, and R. K. Jain. Interstitial hypertension in head and neck tumors in patients—correlation with tumor size. Cancer Res. 52:1993–1995, 1992.
Guyton, A. C., H. J. Granger, and A. E. Taylor. Interstitial fluid pressure. Physiol. Rev. 51:527–563, 1971.
Guyton, A. C., K. Scheel, and D. Murphree. Interstitial fluid pressure. 3. Its effect on resistance to tissue fluid mobility. Circ. Res. 19:412–419, 1966.
Hassid, Y., E. Furman-Haran, R. Margalit, R. Eilam, and H. Degani. Noninvasive magnetic resonance imaging of transport and interstitial fluid pressure in ectopic human lung tumors. Cancer Res. 66:4159–4166, 2006.
Heilmann, M., C. Walczak, J. Vautier, J. L. Dimicoli, C. D. Thomas, M. Lupu, J. Mispelter, and A. Volk. Simultaneous dynamic T1 and T2* measurement for AIF assessment combined with DCE MRI in a mouse tumor model. MAGMA 20:193–203, 2007.
Herneth, A. M., S. Guccione, and M. Bednarski. Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur. J. Radiol. 45:208–213, 2003.
Jain, R. K. Transport of molecules across tumor vasculature. Cancer Metastasis Rev. 6:559–593, 1987.
Jain, R. K., and L. T. Baxter. Mechanisms of heterogeneous distribution of monoclonal-antibodies and other macromolecules in tumors—significance of elevated interstitial pressure. Cancer Res. 48:7022–7032, 1988.
Khaled, A. R. A., and K. Vafai. The role of porous media in modeling flow and heat transfer in biological tissues. Int. J. Heat Mass Transf. 46:4989–5003, 2003.
Kim, J. H., G. W. Astary, X. Chen, T. H. Mareci, and M. Sarntinoranont. Voxelized model of interstitial transport in the rat spinal cord following direct infusion into white matter. J. Biomech. Eng. 131:071007, 2009.
Linninger, A. A., M. R. Somayaji, T. Erickson, X. Guo, and R. D. Penn. Computational methods for predicting drug transport in anisotropic and heterogeneous brain tissue. J. Biomech. 41:2176–2187, 2008.
Lyng, H., O. Haraldseth, and E. K. Rofstad. Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn. Reson. Med. 43:828–836, 2000.
Maier, C. F., Y. Paran, P. Bendel, B. K. Rutt, and H. Degani. Quantitative diffusion imaging in implanted human breast tumors. Magn. Reson. Med. 37:576–581, 1997.
Milosevic, M., A. Fyles, D. Hedley, M. Pintilie, W. Levin, L. Manchul, and R. Hill. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Res. 61:6400–6405, 2001.
Milosevic, M. F., A. W. Fyles, and R. P. Hill. The relationship between elevated interstitial fluid pressure and blood flow in tumors: a bioengineering analysis. Int. J. Radiat. Oncol. Biol. Phys. 43:1111–1123, 1999.
Nathanson, S. D., and L. Nelson. Interstitial fluid pressure in breast-cancer, benign breast conditions, and breast parenchyma. Ann. Surg. Oncol. 1:333–338, 1994.
Netti, P. A., D. A. Berk, M. A. Swartz, A. J. Grodzinsky, and R. K. Jain. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 60:2497–2503, 2000.
Paulson, O. B., M. M. Hertz, T. G. Bolwig, and N. A. Lassen. Filtration and diffusion of water across the blood–brain barrier in man. Microvasc. Res. 13:113–124, 1977.
Pozrikidis, C., and D. A. Farrow. A model of fluid flow in solid tumors. Ann. Biomed. Eng. 31:181–194, 2003.
Pusenjak, J., and D. Miklavcic. Modeling of interstitial fluid pressure in solid tumor. Simul. Pract. Theory 8:17–24, 2000.
Radjenovic, A., J. P. Ridgway, and M. A. Smith. A method for pharmacokinetic modelling of dynamic contrast enhanced MRI studies of rapidly enhancing lesions acquired in a clinical setting. Phys. Med. Biol. 51:N187–N197, 2006.
Rozijn, T. H., B. P. van der Sanden, A. Heerschap, J. H. Creyghton, and W. M. Bovee. Determination of in vivo rat muscle Gd-DTPA relaxivity at 6.3 T. MAGMA 9:65–71, 1999.
Sarntinoranont, M., X. M. Chen, J. B. Zhao, and T. H. Mareci. Computational model of interstitial transport in the spinal cord using diffusion tensor imaging. Ann. Biomed. Eng. 34:1304–1321, 2006.
Sevick, E. M., and R. K. Jain. Measurement of capillary filtration coefficient in a solid tumor. Cancer Res. 51:1352–1355, 1991.
Smith, J. H., and J. A. C. Humphrey. Interstitial transport and transvascular fluid exchange during infusion into brain and tumor tissue. Microvasc. Res. 73:58–73, 2007.
Stohrer, M., Y. Boucher, M. Stangassinger, and R. K. Jain. Oncotic pressure in solid tumors is elevated. Cancer Res. 60:4251–4255, 2000.
Swabb, E. A., J. Wei, and P. M. Gullino. Diffusion and convection in normal and neoplastic tissues. Cancer Res. 34:2814–2822, 1974.
Swartz, M. A., and M. E. Fleury. Interstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 9:229–256, 2007.
Tan, W. H. K., F. J. Wang, T. Lee, and C. H. Wang. Computer simulation of the delivery of etanidazole to brain tumor from PLGA wafers: comparison between linear and double burst release systems. Biotechnol. Bioeng. 82:278–288, 2003.
Tofts, P. S., and A. G. Kermode. Measurement of the blood–brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn. Reson. Med. 17:357–367, 1991.
Truskey, G. A., F. Yuan, and D. F. Katz. Transport Phenomena in Biological Systems. Upper Saddle River: Pearson Prentice Hall, 2004; (710 pp).
Victorino, G. P., R. M. Ramirez, T. J. Chong, B. Curran, and J. Sadjadi. Ischemia-reperfusion injury in rats affects hydraulic conductivity in two phases that are temporally and mechanistically separate. Am. J. Physiol. Heart C. 295:H2164–H2171, 2008.
Weinmann, H. J., M. Laniado, and W. Mutzel. Pharmacokinetics of Gddtpa dimeglumine after intravenous-injection into healthy-volunteers. Physiol. Chem. Phys. 16:167–172, 1984.
Young, J. S., C. E. Lumsden, and A. L. Stalker. The significance of the tissue pressure of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. J. Pathol. Bacteriol. 62:313–333, 1950.
Zhao, J., H. Salmon, and M. Sarntinoranont. Effect of heterogeneous vasculature on interstitial transport within a solid tumor. Microvasc. Res. 73:224–236, 2007.
Acknowledgments
We would like to thank Dr. Dietmar Siemann, Dr. Lori Rice, and Chris Pampo for providing the murine KHT sarcoma cells and tumor inoculation. This research was funded by the University of Florida’s Research and Graduate Programs Opportunity Fund and a grant from the National Institutes of Health (R21 NS05270). MR data was obtained at the Advanced Magnetic Resonance Imaging and Spectroscopy facility in the McKnight Brain Institute of the University of Florida.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jeffrey L. Duerk oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Pishko, G.L., Astary, G.W., Mareci, T.H. et al. Sensitivity Analysis of an Image-Based Solid Tumor Computational Model with Heterogeneous Vasculature and Porosity. Ann Biomed Eng 39, 2360–2373 (2011). https://doi.org/10.1007/s10439-011-0349-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-011-0349-7