Abstract
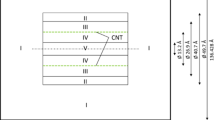
A combination of ab initio quantum mechanical (QM) calculations and canonical Monte Carlo (CMC) simulations are employed to investigate possible usage of single-walled silicon nanotubes (SWSiNTs) as a novel media for hydrogen and methane adsorption as well as their separation from each other. By fitting the force field, a Morse potential model is selected as an efficient potential to describe the binding energies between both hydrogen-SiNTs and methane-SiNTs obtained from ab initio calculations. Then CMC simulations are performed to evaluate the adsorption and separation behaviors of H2 and CH4 on the three different sizes of SiNTs including (5, 5), (7, 7), and (9, 9) SiNTs at ambient temperatures and pressures from 1 up to 10 MPa. As a comparison, the adsorption and separation of H2 and CH4 on the (8, 8) CNTs which are isodiameter with (5, 5) SiNTs are also simulated. Results are indicative of remarkable enhancement of H2 and CH4 adsorption capacity on the SiNTs compared to the CNTs, which arise from stronger van der Waals (VDW) attractions. In the case of methane adsorption on SiNTs, the stored volumetric energy exceeds the goal of the US Freedom CAR Partnership by 2010, which can not be achieved by methane compression at such low pressures. Moreover, simulation results indicate that SiNTs preferentially adsorb methane relative to hydrogen in their equimolar mixture, which results in efficient separation of these gases from each other at 293 K.
Similar content being viewed by others
References
Bekyarova, E., Murata, K., Yudasaka, M., Kasuya, D., Iijima, S., Tanaka, H., Kahoh, H., Kaneko, K.: Single-wall nanostructured carbon for methane storage. J. Phys. Chem. B 107, 4681–4684 (2003)
Bhatia, S.K., Myers, A.L.: Optimum conditions for adsorptive storage. Langmuir 22, 1688–1700 (2006)
Cao, D., Wang, W., Duan, X.: Grand canonical Monte Carlo simulation for determination of optimum parameters for adsorption of supercritical methane in pillared layered pores. J. Colloid Interface Sci. 254, 1–7 (2002)
Cao, D., Zhang, X., Chen, J., Wang, W., Yun, J.: Optimization of single-walled carbon nanotube arrays for methane storage at room temperature. J. Phys. Chem. B 107, 13286–13292 (2003)
Chen, H., Sholl, D.S.: Predictions of selectivity and flux for CH4/H2 separations using single walled carbon nanotubes as membranes. J. Membr. Sci. 269, 152–160 (2006)
Chen, Y.W., Tang, Y.H., Pei, L.Z., Guo, C.: Self-assembled silicon nanotubes grown from silicon monoxide. Adv. Mater. 17, 564–567 (2005)
Cote, A.P., Benin, A.I., Ockwig, N.W., O’Keeffe, M., Matzger, A.J., Yaghi, O.M.: Porous, crystalline, covalent organic framework. Science 310, 1166–1170 (2005)
Dillon, A.C., Jones, K.M., Bekkedahl, T.A., Kiang, C.H., Bethune, D.S., Heben, M.J.: Storage of hydrogen in single-walled carbon nanotubes. Nature 386, 377–379 (1997)
Estela-Uribe, J.F., Jaramillo, J., Salazar, M.A., Trusler, J.P.M.: Virial equation of state for natural gas systems. Fluid Phase Equilib. 204, 169–182 (2003)
Gu, C., Gao, G.-H., Yu, Y.-X., Nitta, T.: Simulation for separation of hydrogen and carbon monoxide by adsorption on single-walled carbon nanotubes. Fluid Phase Equilib. 194–197, 297–307 (2002)
Heyden, A., Düren, T., Keil, J.F.: Study of molecular shape and non-ideality effects on mixture adsorption isotherms of small molecules in carbon nanotubes: a grand canonical Monte Carlo simulation study. Chem. Eng. Sci. 57, 2439–2448 (2002)
Huang, L., Zhang, L., Shao, Q., Lu, L., Lu, X., Jiang, S., Shen, W.: Simulations of binary mixture adsorption of carbon dioxide and methane in carbon nanotubes: temperature, pressure, and pore size effects. J. Phys. Chem. C 111, 11912–11920 (2007)
Kleiner, A., Eggert, S.: Curvature, hybridization, and STM images of carbon nanotubes. Phys. Rev. B 64, 113402 (2001)
Kowalczyk, P., Solarz, L., Do, D.D., Samborski, A., MacElroy, J.M.D.: Nanoscale tubular vessels for storage of methane at ambient temperatures. Langmuir 22, 9035–9040 (2006)
Kowalczyk, P., Brualla, L., Zywociński, A., Bhatia, S.K.: Single-walled carbon nanotubes: efficient nanomaterials for separation and on-board vehicle storage of hydrogen and methane mixture at room temperature? J. Phys. Chem. C 111, 5250–5257 (2007)
Lan, J., Cheng, D., Cao, D., Wang, W.: Silicon nanotube as a promising candidate for hydrogen storage: from the first principle calculations to grand canonical Monte Carlo simulations. J. Phys. Chem. C 112, 5598–5604 (2008)
Lee, J.-W., Kang, H.-C., Shim, W.-G., Kim, C., Moon, H.: Methane adsorption on multi-walled carbon nanotube at (303.15, 313.15, and 323.15) K. J. Chem. Eng. Data 51, 963–967 (2006)
Li, Y., Yang, R.T.: Significantly enhanced hydrogen storage in metal-organic frameworks via spillover. J. Am. Chem. Soc. 128, 726–727 (2005)
Li, Y., Yang, R.T.: Hydrogen storage in metal-organic frameworks by bridged hydrogen spillover. J. Am. Chem. Soc. 128, 8136–8137 (2006)
Lithoxoos, G.P., Samios, J., Carissan, Y.: Investigation of silicon model nanotubes as potential candidate nanomaterials for efficient hydrogen storage: a combined ab initio/grand Canonical Monte Carlo simulation study. J. Phys. Chem. C 112, 16725–16728 (2008)
Meng, T.D., Wang, C.-Y., Wang, S.-Y.: First-principles study of a single Ti atom adsorbed on silicon carbide nanotubes and the corresponding adsorption of hydrogen molecules to the Ti atom. Chem. Phys. Lett. 437, 224–228 (2007)
Morales-Cas, A.M., Moya, C., Coto, B., Vega, L.F., Calleja, G.: Adsorption of hydrogen and methane mixtures on carbon cylindrical cavities. J. Phys. Chem. C 111, 6473–6480 (2007)
Mpourmpakis, G., Froudakis, G.E., Lithoxoos, G.P., Samios, J.: SiC nanotubes: a novel material for hydrogen storage. Nano Lett. 6, 1581–1583 (2006)
Ohkubo, T., Miyawaki, J., Kaneko, K., Ryoo, R., Seaton, N.A.: Adsorption properties of templated mesoporous carbon (CMK-1) for nitrogen and supercritical methane experiment and GCMC simulation. J. Phys. Chem. B 106, 6523–6528 (2002)
Peng, X., Cao, D., Wang, W.: Heterogeneity characterization of ordered mesoporous carbon adsorbent CMK-1 for methane and hydrogen storage: GCMC simulation and comparison with experiment. J. Phys. Chem. C 112, 13024–13036 (2008)
Poirier, E., Chahine, R., Benard, P., Lafi, L., Dorval-Douville, G., Chandonia, P.A.: Hydrogen adsorption measurements and modeling on metal-organic frameworks and single-walled carbon nanotubes. Langmuir 22, 8784–8789 (2006)
Rosi, N.L., Eckert, J., Eddaoudi, M., Vodak, D.T., Kim, J., O’Keeffe, M., Yaghi, O.M.: Hydrogen storage in microporous metal-organic frameworks. Science 300, 1127–1129 (2003)
Roussel, T., Pellenq, R.J.M., Bienfait, M., Vix-Guterl, C., Gadiou, R., Beguin, F., Johnson, M.: Thermodynamic and neutron scattering study of hydrogen adsorption in two mesoporous ordered carbons. Langmuir 22, 4614–4619 (2006)
Ryou, J., Hong, S., Kim, G.: Hydrogen adsorption on hexagonal silicon nanotubes. Solid State Commun. 148, 469–471 (2008)
Sha, J., Niu, J., Ma, X., Xu, J., Zhang, X., Yang, Q., Yang, D.: Silicon nanotubes. Adv. Mater. 14, 1219–1221 (2002)
Tanaka, H., Kanoh, H., Yudasaka, M., Iijima, S., Kaneko, K.: Quantum effects on hydrogen isotope adsorption on single-wall carbon nanohorns. J. Am. Chem. Soc. 127, 7511–7516 (2005)
Tang, Y.H., Pei, L.Z., Chen, Y.W., Guo, C.: Self-assembled silicon nanotubes under supercritically hydrothermal conditions. Phys. Rev. Lett. 95, 116102 (2005)
Yaghi, O.M., O’Keeffe, M., Ockwig, N.W., Chae, H.K., Eddaoudi, M., Kim, J.: Reticular synthesis and the design of new materials. Nature 423, 705–714 (2003)
Zhang, Y.: Computational study of the transport mechanisms of molecules and ions in solid materials. Dissertation, Texas A&M University, Texas, USA (2006)
Zhou, L., Sun, Y., Yang, Z., Zhou, Y.: Hydrogen and methane sorption in dry and water-loaded multiwall carbon nanotubes. J. Colloid Interface Sci. 289, 347–351 (2005)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balilehvand, S., Hashemianzadeh, S.M., Razavi, S. et al. Investigation of hydrogen and methane adsorption/separation on silicon nanotubes: a hierarchical multiscale method from quantum mechanics to molecular simulation. Adsorption 18, 13–22 (2012). https://doi.org/10.1007/s10450-011-9375-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-011-9375-x