Abstract
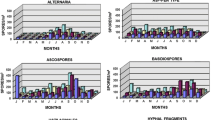
The orchid collection of the ELTE Botanical Garden, Budapest, Hungary was monitored for airborne fungi using viable and non-viable air-sampling methods (Hirst-type and a 3-stage Andersen sampler) with three different culture media. A new culture method was also applied to identify fungal spores from Hirst-type samples. The aim of this study was to determine the diversity, human- and phytopathological potential of the air spora. To find out sources of airborne fungi, samples were collected from the air in an adjacent greenhouse and outdoors, and from necrotic plants. A total of 58 genera were found in the air samples. Cladosporium and Penicillium spp. were common members of the airborne biota. A high proportion (27.5%) of identified genera may be presented as a member of microbial consortium associated with the orchids. Airborne fungi potentially pathogenic to humans were also detected. One species, Zygosporium masonii, was new to Hungary. Statistical analysis indicated that conditions of sampling had significant effects. The principal component analysis elucidated the three principal components representing 75.34% of the total variance; the clusters of variables were related to the three types of culture media. Relative abundance of small-sized spores was high, presumably because of the fungal species composition and accelerated sedimentation of large spores in still air. Apparently, in the studied orchid greenhouse, a specific mycobiota developed due to the climate and hosts (Orchideaceae) grown there.


Similar content being viewed by others
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Andersen, A. A. (1958). A new sampler for the collection, sizing and enumeration of viable airborne bacteria. Journal of Bacteriology, 76, 471–484.
Askew, D. J., & Laing, M. D. (1993). An adapted selective medium for the quantitative isolation of Trichoderma species. Plant Pathology, 42, 686–690.
Atlas, R. M. (1946). Handbook of microbial media (3rd ed.). Boca Raton: CRC Press.
Bayman, P., & Tupac Otero, J. (2006). Microbial endophytes of orchid roots. In B. Schulz, C. Boyle, & T. Sieber (Eds.), Microbial root endophytes (pp. 153–173). New York: Springer.
Bernard, N. (1909). L’évolution dans la symbiose. Le orchidées et leurs champignons commensaux. Annals de Sciences Naturelles Botanique, 9, 1–196.
Blomquist, G., & Andersson, B. (1994). Measurements of microorganisms in non-industrial environments in northern Sweden. In R. A. Samson, B. Flanningan, M. E. Flanningan, A. P. Verhoeff, O. C. G. Adan, & E. S. Hoekstra (Eds.), Health impaction of fungi in indoor environments. Air Quality Monographs 2 (pp. 39–47). Amsterdam: Elsevier Science.
Booth, C. (1971). The genus Fusarium. London: CMI, Eastern Press.
Brundrett, M. C. (2007). Scientific approaches to Australian temperate terrestrial orchid conservation. Australian Journal of Botany, 55, 293–307.
Currah, R. S., Zelmer, C. D., Hambleton, S., & Richardson, K. A. (1997). Fungi from orchid mycorrhizas. In J. Arditti & A. Pridgeon (Eds.), Orchid Biology: Reviews and perspectives Volume 7 (pp. 117–170). Lancaster: Kluwer Academic Publishers.
Divakaran, M., Pillai, G. S., Babu, K. N., & Peter, K. V. (2008). Isolation and fusion of protoplasts in Vanilla species. Current Science, 94(1), 115–120.
Ellis, M. B. (1971). Dematiaceous Hyphomycetes. Kew: Commonwealth Mycological Institute.
Feller, W. (1950). An introduction to probability theory and its applications. New York: Wiley.
Fitton, M., & Holliday, P. (1970). Myrothecium roridum. C.M.I. Descriptions of Pathogenic Fungi and Bacteria No. 253. Ferry Lane: Commonwealth Mycological Institute.
Friedrich, S., Gebelein, D., & Boyle, C. (2005). Control of Botrytis cinerea in glasshouse fuchsia by specific climate management. European Journal of Plant Pathology, 111, 249–262.
Frinking, H. D. (1991). Aerobiology of ‘closed’ agricultural systems. Grana, 30, 481–485.
Frinking, H. D., & Scholte, B. (1983). Dissemination of mildew spores in a glasshouse. Philosophical Transactions of the Royal Society of London, B, 302, 575–582.
Frinking, H. D., Gorissen, A., & Verheul, M. J. (1987). Dissemination of spores in a glasshouse: Pattern or chaos? International Journal of Biometeorology, 31, 147–156.
Gregory, P. H. (1961). The Microbiology of the Atmosphere. London: Leonard Hill.
Harman, G. E., Howell, C. R., Viterbo, A., Chet, I., & Lorito, M. (2004). Trichoderma species-opportunistic, avirulent plant symbionts. Nature reviews. Microbiology, 2(1), 43–56.
Hausbeck, M. K., & Pennypacker, S. P. (1991). Influence of grower activity on concentrations of airborne conidia of Botrytis cinerea among geranium cuttings. Plant Disease, 75, 1236–1243.
Hirst, J. M. (1952). An automatic volumetric spore trap. Annals of Applied Biology, 39, 257–265.
Hislop, E. C. (1969). Splash dispersal of fungus spores and fungicides in the laboratory and greenhause. Annals of Applied Biology, 63, 71–80.
Illyés, Z., Halász, K., Rudnóy, S., Ouanphanivanh, N., Garay, T., & Bratek, Z. (2009). Changes in the diversity of the mycorrhizal fungi of orchids as a function of the water supply of the habitat. Journal of Applied Botany and Food Quality, 83(28), 28–36.
Jarvis, W. R. (1962). The dispersal of spores of Botrytis cinerea Fr. in a raspberry plantation. Transactions of the British mycological Society, 45, 549–559.
Jensen, D. F., Knudsen, I. M. B., Lubeck, M., Mamarabadi, M., Hockenhull, J., & Jensen, B. (2007). Development of a biocontrol agent for plant disease control with special emphasis on the near commercial fungal antagonist Clonostachys rosea strain `IK726’. Australasian Plant Pathology, 36(2), 95–101.
Kerssies, A. (1993a). Horizontal and vertical distribution of airborne conidia of Botrytis cinerea in gerbera crop grown under glass. Netherlands Journal of Plant Pathology, 99, 303–311.
Kerssies, A. (1993b). Influence of environmental conditions on dispersal of Botrytis cinerea conidia and on post-harvest inferction of gerbera flowers grown under glass. Plant Pathology, 42, 754–762.
Klich, M. A. (2002). Identification of common Aspergillus species. Utrecht: Centraalbureau voor Schimmelcultures.
Ko, W. H., & Hora, F. K. (1971). A selective medium for the quantitative determination of Rhizoctonia solani in soil. Phytopathology, 61, 707–710.
Li, D.-W., & LaMondia, J. (2009). Airborne fungi associated with ornamental plant propagation in greenhouses. Aerobiologia, 26(1), 15–28.
Li, D.-W., & Yang, C. S. (2004). Notes on indoor fungi I: New records and noteworthy fungi from indoor environments. Mycotaxon, 89(2), 473–488.
Magyar, D., Barasits, T., Fischl, G., & Fernando, W. G. D. (2006). First record of the natural occurrence of the teleomorph of Leptosphaeria maculans on oilseed rape and airborne dispersal of ascospores in Hungary. Journal of Phytopathology, 154, 428–431.
Magyar, D., Frenguelli, G., Bricchi, E., Tedeschini, E., Csontos, P., Li, D.-W., et al. (2009). The biodiversity of air spora in an Italaian vineyard. Aerobiologia, 25, 99–109.
Miura, K., & Kudo, Y. M. (1970). An agar-medium for aquatic Hyphomycetes. Transactions of the Mycological Society of Japan, 11, 116–118.
Monsó, E., Magarolas, R., Badorrey, I., Radon, K., Nowak, D., & Morera, J. (2002). Occupational asthma in greenhouse flower and ornamental plant growers. American Journal of Respiratory and Critical Care Medicine, 165(7), 954–960.
Nirenberg, H. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Section Liseola. Mitteilungen aus der Biologische Bundesanstalt für Land-und Forstwirtschaft, Berlin-Dahlem, 169, 1–117.
Rajasab, A. H., & Ramalingam, A. (1989). Splash dispersal in Colletotrichum graminicola (Ces.) Wilson, the causal organism of anthracnose of sorghum. Proceedings of the Indian Academy of Sciences, 99(5), 445–451.
Richardson, K., Currah, R., & Hambleton, S. (1993). Basidiomycetous endophytes from the roots of neotropical epiphytic Orchideaceae. Lindleyana, 8(3), 127–137.
Rodolfi, M., Lorenzi, E., & Picco, A. M. (2003). Study of the occurrence of greenhouse microfungi in a Botanical Garden. Journal of Phytopathology, 151(11–12), 591–599.
Rossi, V., Pattori, E., Languasco, L., & Giousè, S. (2000). Dispersal of Fusarium species causing head blight of winter wheat under field conditions. In H. I. Nierenberg (Ed.), Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft, Berlin-Dahlem, 377, 6th European Fusarium seminar and third COST 835 workshop of agriculturally important toxigenic fungi (pp. 45–46). Berlin: Parey Buchverlag.
Samson, R. A., Hoekstra, E. S., Frisvad, J. C., & Filtenborg, O. (2000). Introduction to food—and airborne fungi. Utrecht: Centraalbureau Voor Schimmelcultures.
Schepers, H. T. A. M. (1984). A pattern in the appearence of cucumber powdery mildew in Duch glasshouses. Netherlands Journal of Plant Pathology, 90, 247–256.
Sutton, J. C. (1982). Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Canadian Journal of Plant Pathology, 4, 195–209.
Sutton, J. C., Swanton, C. J., & Gillespie, T. J. (1978). Relation of weather variables and host factors to incidence of airborne spores of Botrytis squamosa. Canadian Journal of Botany, 56, 2460–2469.
Taubenhaus, J. J. (1920). Diseases of greenhouse crops and their control. New York: E. P. Dutton.
Tsavkelova, E. A., Cherdyntseva, T. A., Lobakova, E. S., Kolomeitseva, G. L., & Netrusov, A. I. (2001). Microbiota of the Orchid Rhizoplane. Microbiology, 70(4), 492–497.
Tupac Otero, J., Ackerman, J. D., & Bayman, P. (2002). Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids. American Journal of Botany, 89, 1852–1858.
Vajna, L. (1987). Új adatok két ismert gombafaj mikoparazitikus tulajdonságairól. (in Hungarian). Mikológiai Közlemények, 26(2–3), 99–101.
Walkey, D. G., & Harvey, R. (1966). Spore discharge rhythms in Pyrenomycetes. A survey of the periodicity of spore discharge in Pyrenomycetes. Transactions of the British mycological Society, 49(4), 583–592.
Wang, S., Boulard, T., & Haxaire, R. (1999). Air speed profiles in a naturally ventilated greenhouse with a tomato crop. Agricultural and Forest Meteorology, 96(4), 181–188.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. H. Innes, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols (pp. 315–322). San Diego, CA: Academic Press.
World Health Organization Regional Office for Europe. (2009). WHO Guidelines for Indoor Air Quality. Dampness and mould. Copenhagen: WHO Regional Office for Europe.
Yuan, Z., Chen, Y., & Yang, Y. (2009). Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): estimation and characterization. World Journal of Microbiology & Biotechnology, 25(2), 295–303.
Zadoks, J. C. (1967). International dispersal of fungi. Netherlands Journal of Plant Pathology, 73(1), 61–80.
Acknowledgments
The authors are grateful to Dr. László Vajna (Plant Protection Institute of the Hungarian Academy of Science) for his help in the identification of the Coelomycetes and to Dr. Zoltán Naár for identification of the Trichoderma spp. This work was supported by the Hungarian Scientific Research Fund (OTKA) F67908 and K67688.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Magyar, D., Eszéki, E.R., Oros, G. et al. The air spora of an orchid greenhouse. Aerobiologia 27, 121–134 (2011). https://doi.org/10.1007/s10453-010-9182-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-010-9182-y