Abstract
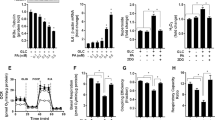
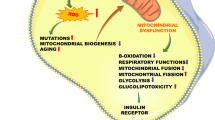
Mitochondrial dysfunction in skeletal muscle has been implicated in the development of insulin resistance, a major characteristic of type 2 diabetes. There is evidence that oxidative stress results from the increased production of reactive oxygen species and reactive nitrogen species leads to mitochondrial dysfunction, tissue damage, insulin resistance, and other complications observed in type 2 diabetes. It has been suggested that intake of high fructose contributes to insulin resistance and other metabolic disturbances. However, there is limited information about the direct effect of fructose on the mitochondrial function of skeletal muscle, the major metabolic determinant of whole body insulin activity. Here, we assessed the effect of fructose exposure on mitochondria-mediated mechanisms in skeletal muscle cells. Exposure of L6 myotubes to high fructose stimulated the production of mitochondrial reactive oxygen species and nitric oxide (NO), and the expression of inducible NO synthase. Fructose-induced oxidative stress was associated with increased translocation of nuclear factor erythroid 2-related factor-2 to the nucleus, decreases in mitochondrial DNA content and mitochondrial dysfunctions, as evidenced by decreased activities of citrate synthase and mitochondrial dehydrogenases, loss of mitochondrial membrane potential, decreased activity of the mitochondrial respiratory complexes, and impaired mitochondrial energy metabolism. Furthermore, positive Annexin–propidium iodide staining and altered expression of Bcl-2 family members and caspases in L6 myotubes indicated that the cells progressively became apoptotic upon fructose exposure. Taken together, these findings suggest that exposure of skeletal muscle cells to fructose induced oxidative stress that decreased mitochondrial DNA content and triggered mitochondrial dysfunction, which caused apoptosis.











Similar content being viewed by others
References
Lowell BB, Shulman GI (2005) Mitochondrial dysfunction and type 2 diabetes. Science 307:384–387
Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT et al (2009) Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119:573–581
Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, Vidal H, Rieusset J (2008) Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest 118:789–800
Fridlyand LE, Philipson LH (2006) Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes Metab 8:136–145
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440:944–948
Rosen P, Nawroth P, King G, Moller W, Tritschler HJ, Packer L (2001) The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev 17:189–212
Zimmet P, Alberti KG, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414:782–787
Hegarty BD, Cooney GJ, Kraegen EW, Furler SM (2002) Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 51:1477–1484
Krebs M, Roden M (2004) Nutrient-induced insulin resistance in human skeletal muscle. Curr Med Chem 11:901–908
Kelley DE, He J, Menshikova EV, Ritov VB (2002) Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51:2944–2950
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671
Lustig RH, Schmidt LA, Brindis CD (2012) Public health: the toxic truth about sugar. Nature 482:27–29
Rutledge AC, Adeli K (2007) Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev 65:S13–S23
Havel PJ (2005) Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 63:133–157
Kashiwagi A, Obata T, Suzaki M, Takagi Y, Kida Y, Ogawa T et al (1992) Increase in cardiac muscle fructose content in streptozotocin-induced diabetic rats. Metabolism 41:1041–1046
Tilton RG, Chang K, Nyengaard JR, Van den Enden M, Ido Y, Williamson JR (1995) Inhibition of sorbitol dehydrogenase. Effects on vascular and neural dysfunction in streptozocin-induced diabetic rats. Diabetes 44:234–242
Stanhope KL, Havel PJ (2008) Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol 19:16–24
Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K (2010) Fructose: a highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab 299:E685–E894
Kaneto H, Fujii J, Myint T, Miyazawa N, Islam KN, Kawasaki Y et al (1996) Reducing sugars trigger oxidative modification and apoptosis in pancreatic beta-cells by provoking oxidative stress through the glycation reaction. Biochem J 320:855–863
Rebollo A, Roglans N, Alegret M, Laguna JC (2012) Way back for fructose and liver metabolism: bench side to molecular insights. World J Gastroenterol 18:6552–6559
Zhao H, Kalivendi S, Zhang H, Joseph J, Nithipatikom K, Vásquez-Vivar J, Kalyanaraman B (2003) Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med 34:1359–1368
Pick E, Keisari Y (1981) Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages-Induction by multiple non-phagocytic stimuli. Cell Immunol 59:301–318
Green LC, Wagner DA, Goglowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138
Srere PA, Matsuoka Y, Mukherjee A (1973) Inhibition studies of rat citrate synthase. J Biol Chem 248:8031–8035
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Paul MK, Kumar R, Mukhopadhyay AK (2008) Dithiothreitol abrogates the effect of arsenic trioxide on normal rat liver mitochondria and human hepatocellular carcinoma cells. Toxicol Appl Pharmacol 226:140–152
Prathapan A, Vineetha VP, Raghu KG (2014) Protective effect of Boerhaavia diffusa L. against mitochondrial dysfunction in angiotensin II induced hypertrophy in H9c2 cardiomyoblast cells. PLoS ONE 9(4):e96220
Evans JL, Goldfine ID, Maddux BA, Grodsky GM (2003) Are oxidative stress activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52:1–8
Theodore M, Kawai Y, Yang J, Kleshchenko Y, Reddy SP, Villalta F, Arinze IJ (2008) Multiple nuclear localization signals function in the nuclear import of the transcription factor Nrf2. J Biol Chem 283:8984–8994
Michel S, Wanet A, De Pauw A, Rommelaere G, Arnould T, Renard P (2012) Crosstalk between mitochondrial (dys)function and mitochondrial abundance. J Cell Physiol 227:2297–2310
Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssière JL, Petit PX, Kroemer G (1995) Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med 181:1661–1672
Cohen GM (1997) Caspases: the executioners of apoptosis. Biochem J 326:1–16
Tappy L, Lê KA, Tran C, Paquot N (2010) Fructose and metabolic diseases: new findings, new questions. Nutrition 26:1044–1049
Busserolles J, Gueux E, Rock E, Mazur A, Rayssiguier Y (2003) High fructose feeding of magnesium deficient rats is associated with increased plasma triglyceride concentration and increased oxidative stress. Magnes Res 16:7–12
Rachek LI, Grishko VI, Ledoux SP, Wilson GL (2006) Role of nitric oxide-induced mtDNA damage in mitochondrial dysfunction and apoptosis. Free Radic Biol Med 40:754–762
Shigenaga MK, Hagen TM, Ames BN (1994) Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 91:10771–10778
Motohashi H, Yamamoto M (2004) Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10:549–557
Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43:233–260
Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13:76–86
Li J, Lee JM, Johnson JA (2002) Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells. J Biol Chem 277:388–394
Venugopal R, Jaiswal AK (1998) Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 17:3145–3156
Murphy MP (2006) Induction of mitochondrial ROS production by electrophilic lipids: a new pathway of redox signaling? Am J Physiol Heart Circ Physiol 290:H1754–H1755
Grishko V, Rachek L, Musiyenko S, Ledoux SP, Wilson GL (2005) Involvement of mtDNA damage in free fatty acid-induced apoptosis. Free Radic Biol Med 38:755–762
Park SY, Choi GH, Choi HI, Ryu J, Jung CY, Lee W (2005) Depletion of mitochondrial DNA causes impaired glucose utilization and insulin resistance in L6 GLUT4myc myocytes. J Biol Chem 280:9855–9864
Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J (2005) mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA 102:719–724
Green K, Brand MD, Murphy MP (2004) Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53:S110–S118
Brookes PS (2005) Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med 38:12–23
Reznick RM, Shulman GI (2006) The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol 574:33–39
Bergeron R, Ren JM, Cadman KS, Moore IK, Perret P, Pypaert M et al (2001) Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am J Physiol Endocrinol Metab 281:E1340–E1346
Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO (2000) Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol 88:2219–2226
Green DR, Reed JC (1998) Mitochondria and apoptosis. Science 281:1309–1312
Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377
Kroemer G, Reed JC (2000) Mitochondrial control of cell death. Nat Med 6:513–519
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9:47–59
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424
Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ et al (2006) Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 311:847–851
Zimmermann KC, Bonzon C, Green DR (2001) The machinery of programmed cell death. Pharmacol Ther 92:57–70
Ferrari G, Yan CY, Greene LA (1995) N-acetylcysteine (D- and L-stereoisomers) prevents apoptotic death of neuronal cells. J Neurosci 15:2857–2866
Acknowledgments
The authors are thankful for financial support from the Council of Scientific and Industrial Research (CSIR), New Delhi, India in the form of network Project THUNDER (BSC0102) and SMiLE (CSC0111). The authors would also like to thank Dr. Amira Klip for providing L6 cells. NJ, CKM, DRA are supported by Senior Research Fellowship of University Grant Commission (UGC), New Delhi. We are thankful to Mr A.L. Vishwakarma, SAIF Division for flowcytometric analysis. This manuscript bears the CDRI Communication No. 8978.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaiswal, N., Maurya, C.K., Arha, D. et al. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis 20, 930–947 (2015). https://doi.org/10.1007/s10495-015-1128-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-015-1128-y