Abstract
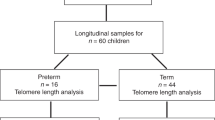
Haemopoietic stem cells (HSC) undergo a process of self renewal to constantly maintain blood cell turnover. However, it has become apparent that adult HSC lose their self-renewal ability with age. Telomere shortening in peripheral blood leukocytes has been seen to occur with age and it has been associated with loss of HSC proliferative capacity and cellular ageing. In contrast foetal HSC are known to have greater proliferative capacity than post-natal stem cells. However it is unknown whether they undergo a similar process of telomere shortening. In this study we show a more accentuated rate of telomere loss in leukocytes from pre term infants compared to human foetuses of comparable age followed longitudinally for 8–12 weeks in a longitudinal study. Our results point to a difference in HSC behaviour between foetal and early postnatal life which is independent of age but may be influenced by events at birth itself.

Similar content being viewed by others
References
Adelman DM, Maltepe E, Simon MC (1999) Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev 13:2478–2483. doi:10.1101/gad.13.19.2478
Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E (2000) Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 14:3191–3203. doi:10.1101/gad.853700
Baxter MA, Wynn RF, Jowitt SN, Wraith JE, Fairbairn LJ, Bellantuono I (2004) Study of telomere length reveals rapid aging of human marrow stromal cells following in vitro expansion. Stem Cells 22(5):675–682
Bellantuono I, Keith WN (2007) Stem cell ageing: does it occur and can we intervene? Expert Rev Mol Med 9:1–20. doi:10.1017/S146239940700049X
Brummendorf TH, Dragowska W, Zijlmans J, Thornbury G, Lansdorp PM (1998) Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J Exp Med 188:1117–1124. doi:10.1084/jem.188.6.1117
Cipolleschi MG, Rovida E, Ivanovic Z, Praloran V, Olivotto M, Dello Sbarba P (2000) The expansion of murine bone marrow cells preincubated in hypoxia as an in vitro indicator of their marrow-repopulating ability. Leukemia 14:735–739. doi:10.1038/sj.leu.2401744
Dando JS, Tavian M, Catelain C, Poirault S, Bennaceur-Griscelli A, Sainteny F, Vainchenker W, Peault B, Lauret E (2005) Notch/Delta4 interaction in human embryonic liver CD34+ CD38- cells: positive influence on BFU-E production and LTC-IC potential maintenance. Stem Cells 23:550–560. doi:10.1634/stemcells.2004-0205
Frenck RW Jr, Blackburn EH, Shannon KM (1998) The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA 95:5607–5610. doi:10.1073/pnas.95.10.5607
Friedrich U, Schwab M, Griese EU, Fritz P, Klotz U (2001) Telomeres in neonates: new insights in fetal hematopoiesis. Pediatr Res 49:252–256. doi:10.1203/00006450-200102000-00020
Ivanovic Z, Dello Sbarba P, Trimoreau F, Faucher JL, Praloran V (2000) Primitive human HPCs are better maintained and expanded in vitro at 1 percent oxygen than at 20 percent. Transfusion 40:1482–1488. doi:10.1046/j.1537-2995.2000.40121482.x
Ivanovic Z, Belloc F, Faucher JL, Cipolleschi MG, Praloran V, Dello Sbarba P (2002) Hypoxia maintains and interleukin-3 reduces the pre-colony-forming cell potential of dividing CD34(+) murine bone marrow cells. Exp Hematol 30:67–73. doi:10.1016/S0301-472X(01)00765-2
Kawanishi S, Oikawa S (2004) Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci 1019:278–284. doi:10.1196/annals.1297.047
Lansdorp PM (1995) Telomere length and proliferation potential of hematopoietic stem cells. J Cell Sci 108(Pt 1):1–6
Lansdorp PM, Dragowska W, Mayani H (1993) Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med 178:787–791. doi:10.1084/jem.178.3.787
Robertson JD, Gale RE, Wynn RF, Dougal M, Linch DC, Testa NG, Chopra R (2000) Dynamics of telomere shortening in neutrophils and T lymphocytes during ageing and the relationship to skewed X chromosome inactivation patterns. Br J Haematol 109:272–279. doi:10.1046/j.1365-2141.2000.01970.x
Rufer N, Brummendorf TH, Kolvraa S, Bischoff C, Christensen K, Wadsworth L, Schulzer M, Lansdorp PM (1999) Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J Exp Med 190:157–167. doi:10.1084/jem.190.2.157
Thornley I, Sutherland R, Wynn R, Nayar R, Sung L, Corpus G, Kiss T, Lipton J, Doyle J, Saunders F, Kamel-Reid S, Freedman M, Messner H (2002) Early hematopoietic reconstitution after clinical stem cell transplantation: evidence for stochastic stem cell behavior and limited acceleration in telomere loss. Blood 99:2387–2396. doi:10.1182/blood.V99.7.2387
Vaughan JI, Manning M, Warwick RM, Letsky EA, Murray NA, Roberts IA (1998) Inhibition of erythroid progenitor cells by anti-Kell antibodies in fetal alloimmune anemia. N Engl J Med 338:798–803. doi:10.1056/NEJM199803193381204
Wynn RF, Cross MA, Hatton C, Will AM, Lashford LS, Dexter TM, Testa NG (1998) Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet 351:178–181. doi:10.1016/S0140-6736(97)08256-1
Yui J, Chiu CP, Lansdorp PM (1998) Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood 91:3255–3262
Acknowledgments
We are very grateful to Bruno Peault, Thomas Von Zglinicki and Ian Thornley for critical reading of the manuscript and helpful discussion. We would like to thank Caroline Wellings, Sue Callan and Joan Kelly for their assistance in patient recruitment to the study and Melissa Baxter, for expert technical assistance with the Southern blotting technique. This study was supported by Royal Manchester Children’s Hospital Endowment Fund. D.K.H. was a Kay Kendall Leukaemia Fund Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10522_2008_9194_MOESM1_ESM.ppt
Analysis of mTRF changes of the same sample run in 3 series of 4–5 adjacent lanes on two different gels to determine the mTRF variability associated with the technique (PPT 31 kb)
10522_2008_9194_MOESM2_ESM.ppt
Representative examples of telomere gels for (A) an alloimmunised foetus. mTRF (kb) for each sample is indicated at the top of each lane. (B) a gel where the same sample was run to assess the variability of the technique (PPT 278 kb)
Rights and permissions
About this article
Cite this article
Holmes, D.K., Bellantuono, I., Walkinshaw, S.A. et al. Telomere length dynamics differ in foetal and early post-natal human leukocytes in a longitudinal study. Biogerontology 10, 279–284 (2009). https://doi.org/10.1007/s10522-008-9194-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-008-9194-y