Abstract
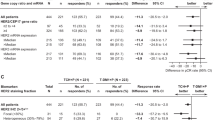
To determine rates of pathologic complete response (pCR) and near-complete response (npCR) in operable early-stage breast cancer using neoadjuvant capecitabine plus docetaxel, with or without trastuzumab, and investigate biomarkers of pathologic response. Women with operable early-stage breast cancer were enrolled in a multicenter study of neoadjuvant therapy for four 21-day cycles with capecitabine 825 mg/m2 plus docetaxel 75 mg/m2 if human epidermal growth factor receptor 2 (HER2)-negative, and additionally, a standard trastuzumab dose if HER2-positive. Primary endpoint was rate of pCR and npCR. Secondary endpoints were potential associations between response and TP53 mutational analysis using the AmpliChip TP53 assay or immunohistochemical (IHC) staining, and genomic subtyping using the PAM50 assay. In patients who completed treatment and surgery, pCR and npCR rates were 15.8% in patients with HER2-negative and 50% in patients with HER2-positive tumors. Stratified by genomic subtype, patients of HER2-enriched subtype had the best response (72.2%), and luminal A (9.1%) and B (4.8%) subtypes, the poorest. Of 147 patients tested for TP53 mutations using the AmpliChip assay, 78 variants were detected; 55 were missense. Response rate among TP53-mutated patients was 30%, significantly higher than TP53 wild-type patients (10%; P = 0.0032). Concordance between AmpliChip mutation status versus TP53 IHC staining was 65%, with AmpliChip status predictive of response and IHC status not predictive. Capecitabine plus docetaxel in HER2-negative, and with trastuzumab in HER2-positive patients, provided a good response rate with four cycles of non-anthracycline-containing therapy. TP53 mutational analysis and genomic subtyping were predictive.


Similar content being viewed by others
References
Mieog JS, Van de Velde CJ (2009) Neoadjuvant chemotherapy for early breast cancer. Expert Opin Pharmacother 10:1423–1434
Knauer M, Haid A, Schneider Y, Köberle-Wührer R, Lang A, Winder T, Alton R, Jasarevic Z, Säly C, Offner FA, Wenzl E, de Vries A (2009) Adjuvant extension of chemotherapy after neoadjuvant therapy may not improve outcome in early-stage breast cancer. Eur J Surg Oncol 35:798–804
Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R, Kahlenberg MS, Paik S, Soran A, Wickerham DL, Wolmark N (2006) Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 24:2019–2027
Heys SD, Hutcheon AW, Sarkar TK, Ogston KN, Miller ID, Payne S, Smith I, Walker LG, Eremin O (2002) Neoadjuvant docetaxel in breast cancer: 3-year survival results from the Aberdeen trial. Clin Breast Cancer 3:S69–S74
von Minckwitz G, Blohmer JU, Raab G, Löhr A, Gerber B, Heinrich G, Eidtmann H, Kaufmann M, Hilfrich J, Jackisch C, Zuna I, Costa SD, German Breast Group (2005) In vivo chemosensitivity-adapted preoperative chemotherapy in patients with early-stage breast cancer: the GEPARTRIO pilot study. Ann Oncol 16:56–63
Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, Ah-See AK, Eremin O, Walker LG, Sarkar TK, Eggleton SP, Ogston KN (2002) Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol 20:1456–1466
von Minckwitz G, Kümmel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD, Jackisch C, Loibl S, Mehta K, Kaufmann M, Group German Breast (2008) Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 100:552–562
von Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kühn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, Untch M (2010) Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol 28:2015–2023
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz AB Jr, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16:2672–2685
Rouzier R, Mathieu MC, Sideris L, Youmsi E, Rajan R, Garbay JR, André F, Marsiglia H, Spielmann M, Delaloge S (2004) Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer 101:918–925
Mauri D, Pavlidis N, Ioannidis JP (2005) Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 97:188–194
Jones S, Holmes FA, O’Shaughnessy J, Blum JL, Vukelja SJ, McIntyre KJ, Pippen JE, Bordelon JH, Kirby RL, Sandbach J, Hyman WJ, Richards DA, Mennel RG, Boehm KA, Meyer WG, Asmar L, Mackey D, Riedel S, Muss H, Savin MA (2009) Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol 27:1177–1183
Crump M, Tu D, Shepherd L, Levine M, Bramwell V, Pritchard K (2003) Risk of acute leukemia following epirubicin-based adjuvant chemotherapy: a report from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 21:3066–3071
Smith RE, Bryant J, DeCillis A, Anderson S, National Surgical Adjuvant Breast and Bowel Project Experience (2003) Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol 21:1195–1204
Lee KS, Ro J, Nam BH, Lee ES, Kwon Y, Kwon HS, Chung KW, Kang HS, Kim EA, Kim SW, Shin KH, Kim SK (2008) A randomized phase-III trial of docetaxel/capecitabine versus doxorubicin/cyclophosphamide as primary chemotherapy for patients with stage II/III breast cancer. Breast Cancer Res Treat 109:481–489
Luck HJ, DuBois A, Schrader I et al (2007) Final results of the AGO breast cancer study group MAMMA-3 trial: first-line capecitabine + paclitaxel vs epirubicin + paclitaxel for high-risk metastatic breast cancer. Breast Cancer Res Treat 106:S67
Wardley AM, Pivot X, Morales-Vasquez F, Zetina LM, de Fátima Dias Gaui M, Reyes DO, Jassem J, Barton C, Button P, Hersberger V, Torres AA (2010) Randomized phase II trial of first-line trastuzumab plus docetaxel and capecitabine compared with trastuzumab plus docetaxel in HER2-positive metastatic breast cancer. J Clin Oncol 28:976–983
Robert NJ, Dieras V, Glaspy J, Brufsky A, Bondarenko I, Lipatov O, Perez E, Yardley D, Zhou X, Phan S (2009) RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC). J Clin Oncol 27:15
Steger GG, Greil R, Jakesz R, Lang A, Mlineritsch B, Melbinger-Zeinitzer E, Marth C, Samonigg H, Kubista E, Gnant M (2009) A randomized phase III study comparing epirubicin, docetaxel, and capecitabine (EDC) to epirubicin and docetaxel (ED) as neoadjuvant treatment for early breast cancer-first results of the Austrian Breast and Colorectal Cancer Study Group-Trial 24 (ABCSG-24). Eur J Cancer Suppl 7:Abstract 4BA
Wildiers H, Neven P, Christiaens MR, Squifflet P, Amant F, Weltens C, Smeets A, van Limbergen E, Debrock G, Renard V, Van Eenoo L, Wynendaele W, Paridaens R (2010) Neoadjuvant capecitabine and docetaxel (plus trastuzumab): effective non-anthracycline-based chemotherapy regimen for patients with locally advanced breast cancer. Ann Oncol
Olivier M, Langerød A, Carrieri P, Bergh J, Klaar S, Eyfjord J, Theillet C, Rodriguez C, Lidereau R, Bièche I, Varley J, Bignon Y, Uhrhammer N, Winqvist R, Jukkola-Vuorinen A, Niederacher D, Kato S, Ishioka C, Hainaut P, Børresen-Dale AL (2006) The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res 12:1157–1167
Norberg T, Lennerstrand J, Inganäs M, Bergh J (1998) Comparison between p53 protein measurements using the luminometric immunoassay and immunohistochemistry with detection of p53 gene mutations using cDNA sequencing in human breast tumors. Int J Cancer 79:376–383
Harris LN, Broadwater G, Lin NU, Miron A, Schnitt SJ, Cowan D, Lara J, Bleiweiss I, Berry D, Ellis M, Hayes DF, Winer EP, Dressler L (2006) Molecular subtypes of breast cancer in relation to paclitaxel response, outcomes in women with metastatic disease: results from CALGB 9342. Breast Cancer Res 8:R66
Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, Quackenbush JF, Stijleman IJ, Palazzo J, Marron JS, Nobel AB, Mardis E, Nielsen TO, Ellis MJ, Perou CM, Bernard PS (2009) Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 27:1160–1167
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, Assad L, Poniecka A, Hennessy B, Green M, Buzdar AU, Singletary SE, Hortobagyi GN, Pusztai L (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414–4422
Grollman AP, Shibutani S, Moriya M, Miller F, Wu L, Moll U, Suzuki N, Fernandes A, Rosenquist T, Medverec Z, Jakovina K, Brdar B, Slade N, Turesky RJ, Goodenough AK, Rieger R, Vukelić M, Jelaković B (2007) Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc Natl Acad Sci USA 104:12129–12134
L’Espérance S, Popa I, Bachvarova M, Plante M, Patten N, Wu L, Têtu B, Bachvarov D (2006) Gene expression profiling of paired ovarian tumors obtained prior to and following adjuvant chemotherapy: molecular signatures of chemoresistant tumors. Int J Oncol 29:5–24
Clopper CJ, Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404
Steger GG, Galid A, Gnant M, Mlineritsch B, Lang A, Tausch C, Rudas M, Greil R, Wenzel C, Singer CF, Haid A, Pöstlberger S, Samonigg H, Luschin-Ebengreuth G, Kwasny W, Klug E, Kubista E, Menzel C, Jakesz R, ABCSG-14 (2007) Pathologic complete response with six compared with three cycles of neoadjuvant epirubicin plus docetaxel and granulocyte colony-stimulating factor in operable breast cancer: results of ABCSG-14. J Clin Oncol 25:2012–2018
Han S, Kim J, Lee J, Chang E, Gwak G, Cho H, Yang KH, Park S, Park K (2009) Comparison of 6 cycles versus 4 cycles of neoadjuvant epirubicin plus docetaxel chemotherapy in stages II and III breast cancer. Eur J Surg Oncol 35:583–587
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, Morandi P, Fan C, Rabiul I, Ross JS, Hortobagyi GN, Pusztai L (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11:5678–5685
Baselga J, Semiglazov V, Manikhas GM, et al (2007) Efficacy of neoadjuvant trastuzumab in patients with inflammatory breast cancer: data from the NOAH (NeOAdjuvant Herceptin) phase III trial. Eur J Cancer Suppl 4:Abstract 2030
Aas T, Børresen AL, Geisler S, Smith-Sørensen B, Johnsen H, Varhaug JE, Akslen LA, Lønning PE (1996) Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat Med 2:811–814
Geisler S, Lønning PE, Aas T, Johnsen H, Fluge O, Haugen DF, Lillehaug JR, Akslen LA, Børresen-Dale AL (2001) Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res 61:2505–2512
Andersson J, Larsson L, Klaar S, Holmberg L, Nilsson J, Inganäs M, Carlsson G, Ohd J, Rudenstam CM, Gustavsson B, Bergh J (2005) Worse survival for TP53 (p53)-mutated breast cancer patients receiving adjuvant CMF. Ann Oncol 16:743–748
Di Leo A, Tanner M, Desmedt C, Paesmans M, Cardoso F, Durbecq V, Chan S, Perren T, Aapro M, Sotiriou C, Piccart MJ, Larsimont D, Isola J, AX T, 303 translational study team (2007) p-53 gene mutations as a predictive marker in a population of advanced breast cancer patients randomly treated with doxorubicin or docetaxel in the context of a phase III clinical trial. Ann Oncol 18:997–1003
Kandioler-Eckersberger D, Ludwig C, Rudas M, Kappel S, Janschek E, Wenzel C, Schlagbauer-Wadl H, Mittlböck M, Gnant M, Steger G, Jakesz R (2000) TP53 mutation and p53 overexpression for prediction of response to neoadjuvant treatment in breast cancer patients. Clin Cancer Res 6:50–56
Carvajal D, Tovar C, Yang H, Vu BT, Heimbrook DC, Vassilev LT (2005) Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors. Cancer Res 65:1918–1924
Learn PA, Yeh IT, McNutt M, Chisholm GB, Pollock BH, Rousseau DL Jr, Sharkey FE, Cruz AB, Kahlenberg MS (2005) HER-2/neu expression as a predictor of response to neoadjuvant docetaxel in patients with operable breast carcinoma. Cancer 103:2252–2260
Sjöström J, Blomqvist C, Heikkilä P, Boguslawski KV, Räisänen-Sokolowski A, Bengtsson NO, Mjaaland I, Malmström P, Ostenstadt B, Bergh J, Wist E, Valvere V, Saksela E (2000) Predictive value of p53, mdm-2, p21, and mib-1 for chemotherapy response in advanced breast cancer. Clin Cancer Res 6:3103–3110
Hamilton A, Larsimont D, Paridaens R, Drijkoningen M, van de Vijver M, Bruning P, Hanby A, Houston S, Treilleux I, Guastalla JP, Van Vreckem A, Sylvester R, Piccart M (2000) A study of the value of p53, HER2, and Bcl-2 in the prediction of response to doxorubicin and paclitaxel as single agents in metastatic breast cancer: a companion study to EORTC 10923. Clin Breast Cancer 1:233–240
Van Poznak C, Tan L, Panageas KS, Arroyo CD, Hudis C, Norton L, Seidman AD (2002) Assessment of molecular markers of clinical sensitivity to single-agent taxane therapy for metastatic breast cancer. J Clin Oncol 20:2319–2326
Acknowledgments
The XeNA study was sponsored by Roche, Nutley, NJ. The authors wish to thank H. Jeffrey Lawrence for help with study design, implementation, and writing; Debu Tripathy for help with design and initiation of the study; and Nancy Patten, Sim Truong, and Aarthi Balasubramanyan for help with implementation, statistical analysis, and writing. Editorial assistance was provided by Insight Medical Communications Inc., New York, NY, which was funded by Roche-Genentech.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Glück, S., Ross, J.S., Royce, M. et al. TP53 genomics predict higher clinical and pathologic tumor response in operable early-stage breast cancer treated with docetaxel-capecitabine ± trastuzumab. Breast Cancer Res Treat 132, 781–791 (2012). https://doi.org/10.1007/s10549-011-1412-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1412-7